Gene mutation site for predicting anti-HER2 therapeutic drug reactivity of patients with breast cancer and application thereof
A technology for drug response and breast cancer, applied in the medical field, can solve problems such as restricting the development of individualized treatment of tumors, and achieve the effects of preventing drug waste, reducing the burden, and reducing the burden on patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
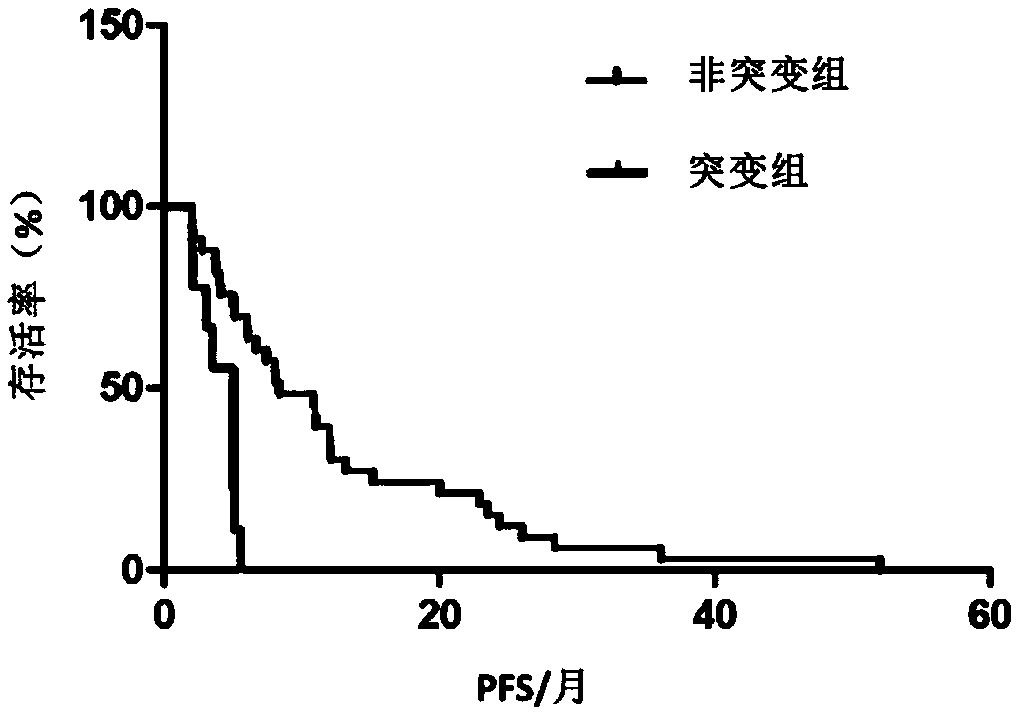
[0052] Example 1 Screening for gene mutation sites associated with drug resistance in breast cancer patients
[0053] 1. Patient and sample collection
[0054] A total of 120 female ABC patients who were being treated in a tumor hospital were collected. These patients were diagnosed as breast cancer by pathological examination; among them, 119 patients had non-invasive ductal carcinoma, and 1 patient had bone marrow carcinoma.
[0055] The level of HER2 positivity was defined by immunohistochemistry 3+, or immunohistochemistry 2+ / fluorescence in situ hybridization+ (FISH+). The breast cancer patients in this study were patients over 18 years old who were diagnosed with metastatic breast cancer. The patient's peripheral blood was collected using streck tubes and centrifuged within 72 hours to separate plasma from peripheral blood cells. This study was approved by the ethics committees of the Chinese Academy of Medical Sciences and the National Cancer Center / Tumor Hospital of ...
Embodiment 2
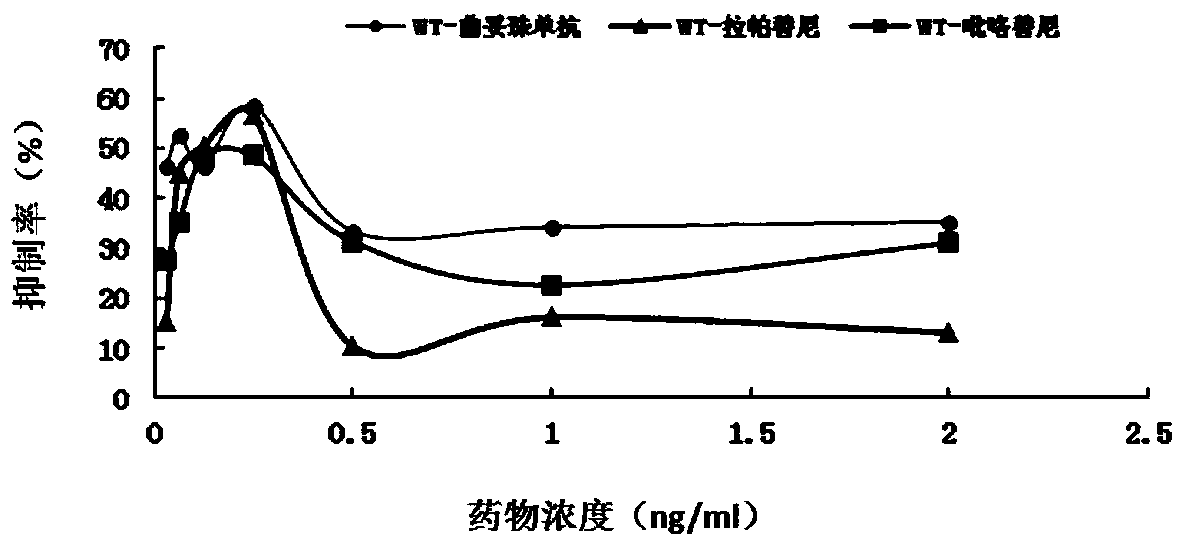
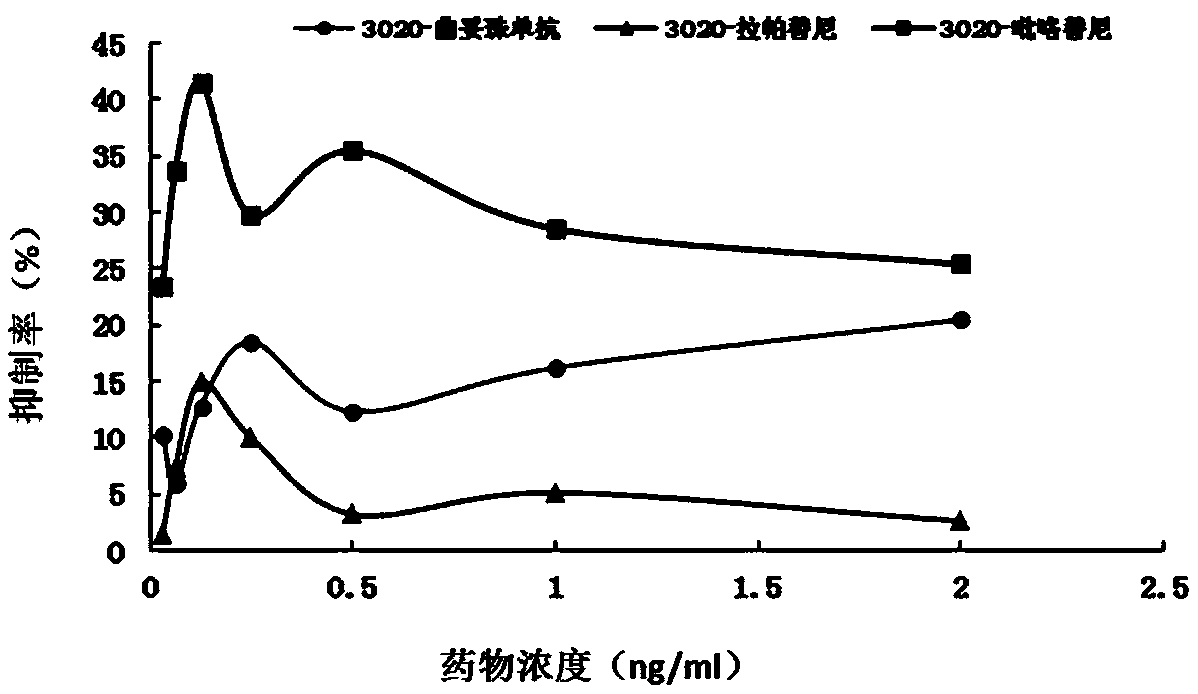
[0074] Example 2 HER2 point mutation c.3020C>T anti-HER2 therapeutic cell test
[0075] 1. Experimental materials
[0076] 1. Strains
[0077] Escherichia coli DH5α, stbl3, is preserved by the State Key Laboratory of Molecular Oncology, Chinese Academy of Medical Sciences (hereinafter referred to as "Key Laboratory").
[0078] 2. Plasmid
[0079] LentiCRISPRV2: Lentiviral expression vector, donated by the laboratory of Dr. Zhang Feng of the Massachusetts Institute of Technology, and its gel recovery product is preserved by the key laboratory.
[0080] LenticrisprV2-HER2: A lentiviral expression vector inserted into the corresponding sgRNA, constructed by this application. (See the experimental method section below for the specific construction method)
[0081] PCMV-GFP: green fluorescent protein lentiviral expression vector, preserved by key laboratories.
[0082] 3. Cell lines
[0083] BT474: Breast cancer cell line, preserved by key laboratories.
[0084] Cell line me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com