Kit and method for detecting CMV infection in trace biological sample of eye
A biological sample and ocular technology, applied in biochemical equipment and methods, microbiological determination/inspection, DNA/RNA fragments, etc., can solve the problem of inability to accurately reflect the real cause of the eye and the lack of high-sensitivity detection of viral infection in ocular tissue Means, unseen issues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1. Method for extracting DNA from test specimens of eye microfluidics
[0118] Material:
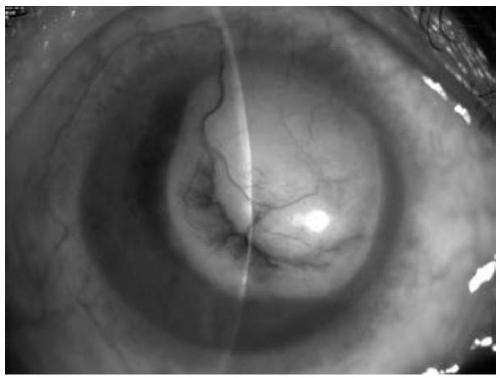
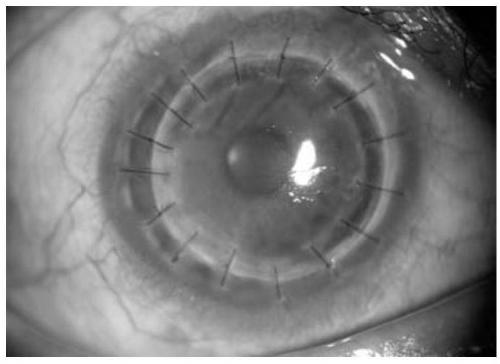
[0119] Specimens to be tested: collected from admitted patients, tear fluid, aqueous humor 2, and vitreous.
[0120] (1) Put the collected sample to be tested into a container, add 10-30 μl proteinase K, 100-300 μl lysis buffer AL, and treat at 56°C for more than 10 minutes;
[0121] (2) Add the same volume of absolute ethanol as the lysis buffer, shake and mix for 10-20s, and centrifuge briefly;
[0122] (3) Put the liquid obtained in step (2) into a spin column, put it into a 2ml collection tube, and centrifuge at 6000-9000rpm for 0.5-2min;
[0123] If the sample volume > 140 μl, repeat step (3);
[0124] (4) Add 300-600μl elution buffer 1, 6000-9000rpm, 0.5-2min, discard the filtrate and collection tube, and replace with a new collection tube;
[0125] (5) Add 300-600μl Elution Buffer 2, centrifuge at 10000-15000rpm for 1-5min, discard the filtrate and collection tu...
Embodiment 2
[0134] Example 2. Method for extracting DNA from eye trace solid specimens to be tested
[0135] Material:
[0136] Specimens to be tested: collected from admitted patients, including retina, corneal endothelium, pterygium, conjunctiva, iris, and eye tumors, with a collection volume of 1×1mm;
[0137] step:
[0138] (1) Put the collected sample to be tested into a container, add 10-30 μl proteinase K, 100-300 μl lysis buffer AL, and treat at 56°C for 6-12 hours;
[0139] (2) Add the same volume of absolute ethanol as the lysis buffer, shake and mix for 10-20s, and centrifuge briefly;
[0140] (3) Put the liquid obtained in step (2) into a spin column, put it into a 2ml collection tube, and centrifuge at 6000-9000rpm for 0.5-2min;
[0141] (4) Add 300-600μl elution buffer 1, 6000-9000rpm, 0.5-2min, discard the filtrate and collection tube, and replace with a new collection tube;
[0142] (5) Add 300-600μl Elution Buffer 2, centrifuge at 10000-15000rpm for 1-5min, discard th...
Embodiment 3
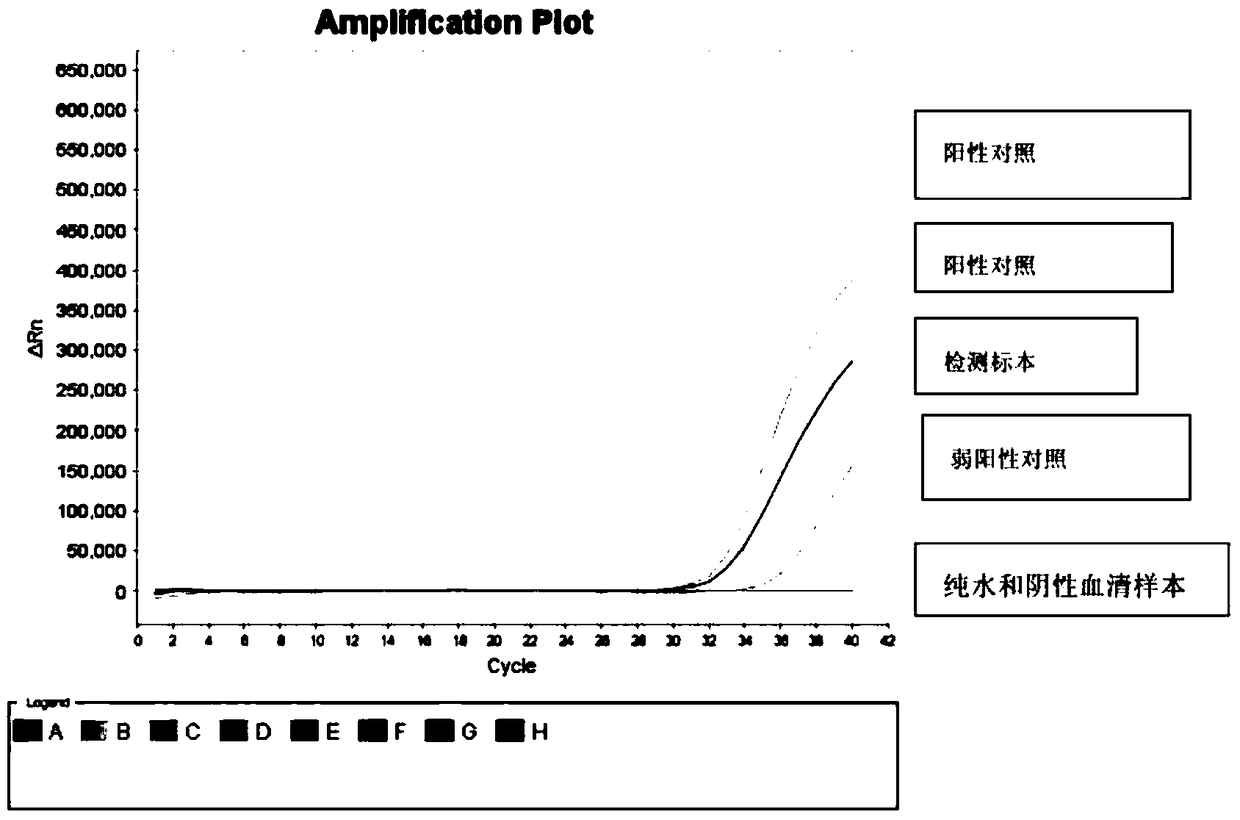
[0150] Example 3. Kit for detection of ocular CMV infection by ocular microsamples
[0151] DNA extraction reagent set:
[0152] Lysis buffer: Tris-saturated phenol with 10% SDS,
[0153] Elution buffer 1: a mixture of saturated phenol: chloroform: isoamyl alcohol with a volume ratio of 25:24:1;
[0154] Elution buffer 2: absolute ethanol,
[0155] Elution buffer 3: pH 8.0, 10 mmol / L Tris-HCl solution containing 1 mmol / LEDTA.
[0156] Specific primers and probes for PCR amplification
[0157] Primer-F: TCGTGAAACACGCTCGCTTT
[0158] Primer-R: TGGTCGGTACACGGTCCCTT
[0159] Probe: GACCCGGTGTTCCTTGGAGA
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com