Kits and methods for detecting CMV infection in ocular trace biological samples
A kit and ocular technology, applied in the field of detection of CMV infection in trace biological samples of the eye, can solve the problems of lack of highly sensitive detection methods for ocular tissue virus infection, unseen, and inability to accurately reflect the real cause of the eye
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
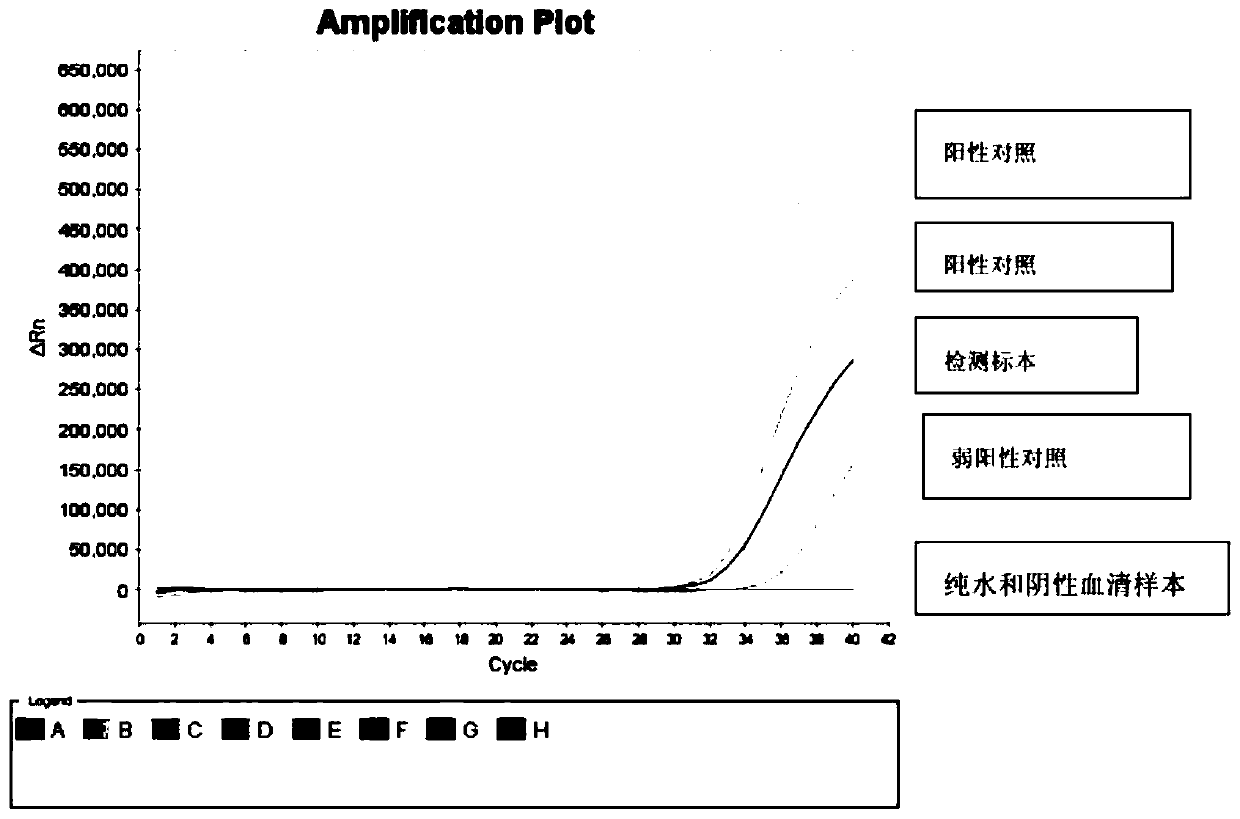
Image
Examples
Embodiment 1
[0118] Example 1. Method for extracting DNA from test specimens of eye microfluidics
[0119] Material:
[0120] Specimens to be tested: collected from admitted patients, tear fluid, aqueous humor 2, and vitreous.
[0121] (1) Put the collected sample to be tested into a container, add 10-30 μl proteinase K, 100-300 μl lysis buffer AL, and treat at 56°C for more than 10 minutes;
[0122] (2) Add the same volume of absolute ethanol as the lysis buffer, shake and mix for 10-20s, and centrifuge briefly;
[0123] (3) Put the liquid obtained in step (2) into a spin column, put it into a 2ml collection tube, and centrifuge at 6000-9000rpm for 0.5-2min;
[0124] If the sample volume > 140 μl, repeat step (3);
[0125] (4) Add 300-600μl elution buffer 1, 6000-9000rpm, 0.5-2min, discard the filtrate and collection tube, and replace with a new collection tube;
[0126] (5) Add 300-600μl Elution Buffer 2, centrifuge at 10000-15000rpm for 1-5min, discard the filtrate and collection tu...
Embodiment 2
[0135] Example 2. Method for extracting DNA from eye trace solid specimens to be tested
[0136] Material:
[0137] Specimens to be tested: collected from admitted patients, including retina, corneal endothelium, pterygium, conjunctiva, iris, and eye tumors, with a collection volume of 1×1mm;
[0138] step:
[0139] (1) Put the collected sample to be tested into a container, add 10-30 μl proteinase K, 100-300 μl lysis buffer AL, and treat at 56°C for 6-12 hours;
[0140] (2) Add the same volume of absolute ethanol as the lysis buffer, shake and mix for 10-20s, and centrifuge briefly;
[0141] (3) Put the liquid obtained in step (2) into a spin column, put it into a 2ml collection tube, and centrifuge at 6000-9000rpm for 0.5-2min;
[0142] (4) Add 300-600μl elution buffer 1, 6000-9000rpm, 0.5-2min, discard the filtrate and collection tube, and replace with a new collection tube;
[0143] (5) Add 300-600μl Elution Buffer 2, centrifuge at 10000-15000rpm for 1-5min, discard th...
Embodiment 3
[0151] Example 3. Kit for detection of ocular CMV infection by ocular microsamples
[0152] DNA extraction reagent set:
[0153] Lysis buffer: Tris-saturated phenol with 10% SDS,
[0154] Elution buffer 1: a mixture of saturated phenol: chloroform: isoamyl alcohol with a volume ratio of 25:24:1;
[0155] Elution buffer 2: absolute ethanol,
[0156] Elution buffer 3: pH 8.0, 10 mmol / L Tris-HCl solution containing 1 mmol / LEDTA.
[0157] Specific primers and probes for PCR amplification
[0158] Primer-F: TCGTGAAACACGCTCGCTTT
[0159] Primer-R: TGGTCGGTACACGGTCCCTT
[0160] Probe: GACCCGGTGTTCCTTGGAGA
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com