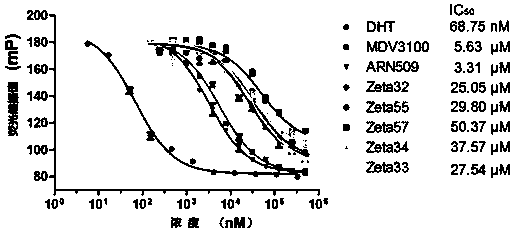
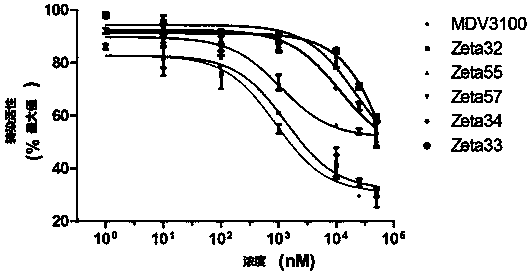
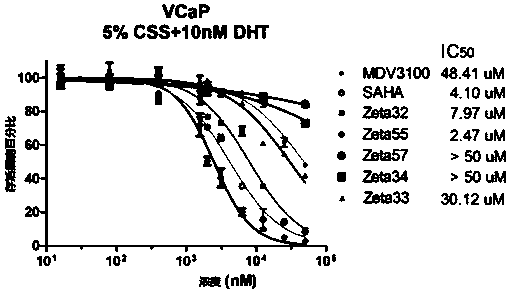
Compound and composition as well as application thereof to drugs preparation
A technology of compounds and compositions, applied in the field of novel antagonist compounds, capable of solving problems such as insignificant ability of prostate cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] Compounds of the present invention have the structure of formula I:
[0037]
[0038] Wherein, R1 is selected from hydrogen, fluorine, chlorine; R2 and R3 are independently selected from hydrogen, alkyl, substituted alkyl, alkenyl or substituted alkenyl, alkynyl or substituted alkynyl, aryl, R2 and R3 can be connected to form a ring which can be cycloalkyl, substituted cycloalkyl, aromatic heterocycle or non-aromatic heterocycle; R4 is selected from hydrogen, cyano, alkyl, substituted alkyl, alkenes Base or substituted alkenyl, alkynyl or substituted alkynyl, aryl; R5 is selected from hydrogen, halogen, haloalkyl.
[0039] The term "alkyl" as used herein denotes a branched or straight hydrocarbon chain, preferably having from about 1 to about 5 carbons, such as methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl , Isobutyl, tert-butyl, 2-methylpentyl, pentyl, etc. "Substituted alkyl" means that the aforementioned alkyl is replaced by one or more functional group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com