Liver disease-related biomarkers and methods of use thereof
A biomarker, liver disease technology, applied in the field of biomarkers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
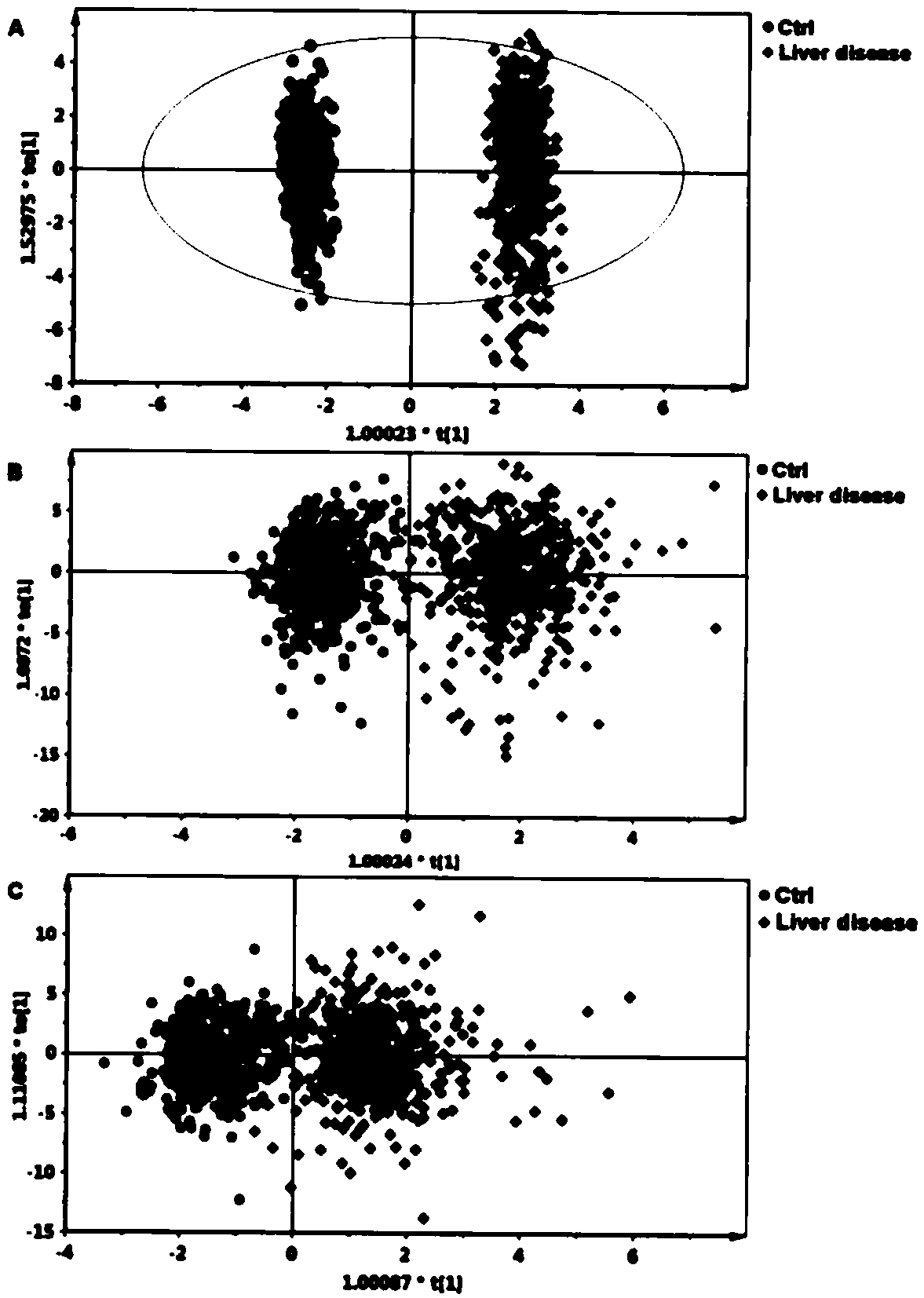
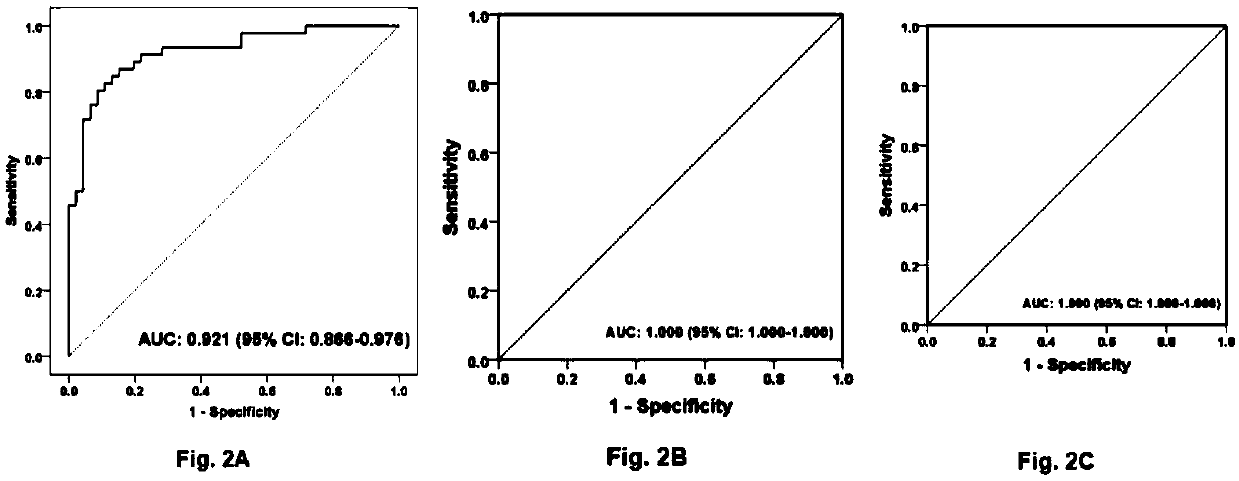
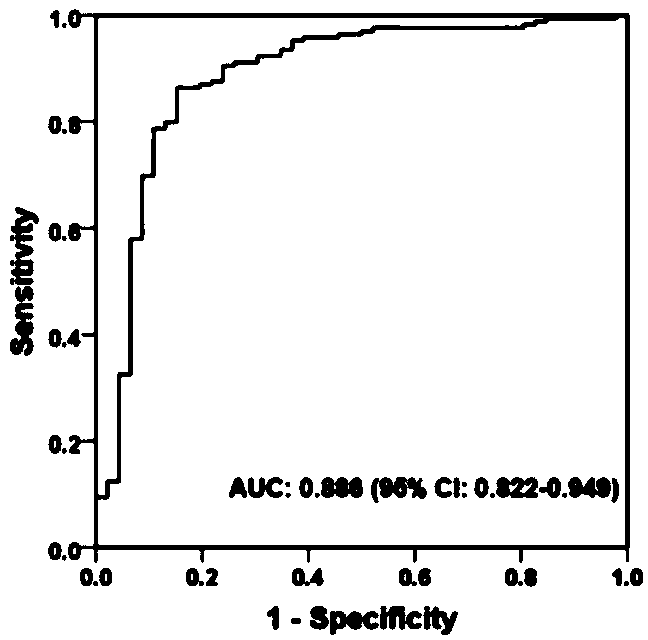
[0267] Example 1 Study population
[0268] Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (Shanghai, China) and Xiamen Hospital of Traditional Chinese Medicine (Xiamen, China) enrolled 504 patients diagnosed with liver fibrosis and cirrhosis, aged 15-75 years. The above diagnosis is based on the "Guidelines for the Prevention and Treatment of Chronic Hepatitis B in China" (Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Hepatology, Chinese Society of Hepatology, Chinese Society of Infectious Diseases, Chinese Medical Association) and "Chinese Chronic Hepatitis B Guidelines for Prevention and Treatment (2005)" (Chinese Journal of Medicine (Engl) 2007; 120:2159-73). Exclusion criteria included: age less than 15 years or greater than 75 years, pregnant or lactating women, infection with human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis D virus (HDV), or hepatitis E virus co-infection (HEV)...
Embodiment 2
[0276] Example 2 liver biopsy
[0277] Except for patients with liver disease diagnosed as decompensated cirrhosis, the remaining patients with liver disease received ultrasound-guided liver biopsy within 1 week after enrollment. Samples were fixed in 10% formalin, embedded in paraffin, and histological sections were stained with hematoxylin and eosin and Masson. A minimum length of at least 1.5 cm and at least 6 portal areas are required for diagnosis. Histological grade of inflammation (G0 to G4) and liver fibrosis according to Scheuer's staging (Scheuer PJ, Standish RA, Dhillon AP. Scoring of chronic hepatitis. Clinics in liver disease 2002; 6:335-47, v-vi.) The stages (S0 to S4). The above classifications were independently and blindly evaluated by three pathologists from Fudan University in Shanghai, China, and the results passed the Kappa consistency test.
Embodiment 3
[0278] Example 3 Serum Sample Collection
[0279] Use LH750 hematology analyzer and Synchron DXC800 clinical system (Beckman Coulter, USA) for hematology and biochemical routine testing. Serum hyaluronic acid (HA) and laminin (LN) concentrations were measured with a chemiluminescent immunoassay (CLIA) system (LUMO, Shinova Systems, Shanghai, China). Coagulation function was measured using an automatic coagulation analyzer (STAGO Compact, Diagnostica Stago, France). Serum HBV-DNA levels were detected using a real-time polymerase chain reaction (PCR) system (LightCycler, 480, Roche, USA).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com