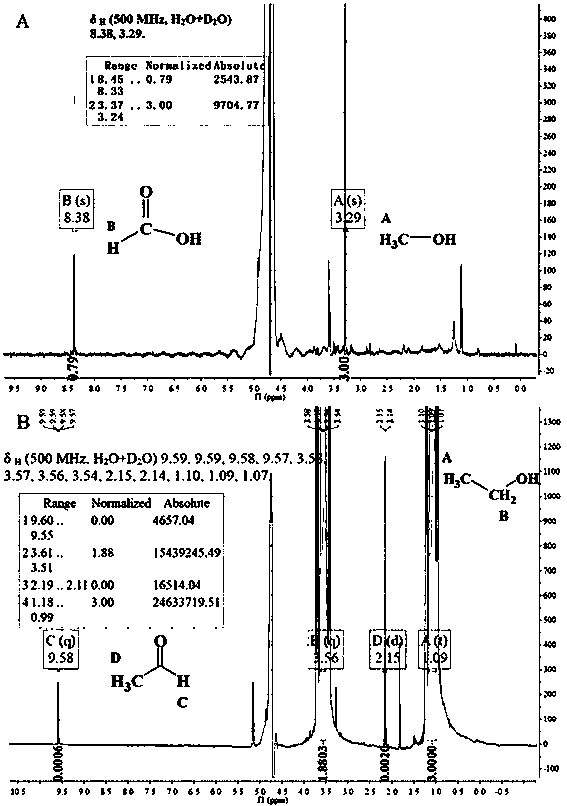
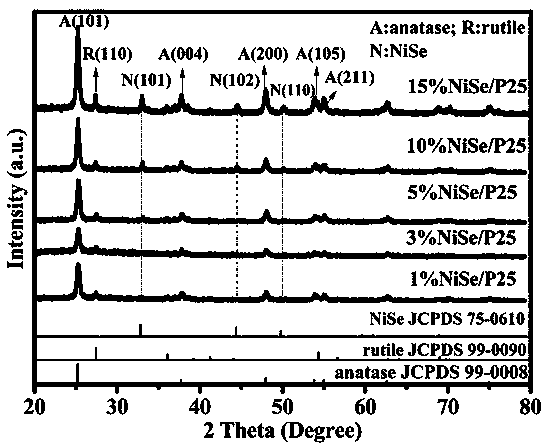
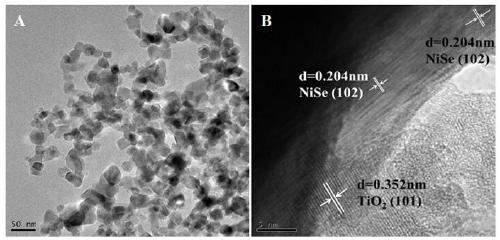
Heterojunction photocatalyst for hydrogen production and alcohol oxidation by high-efficiency photocatalytic water-cracking
A photocatalyst, photocatalytic water technology, applied in physical/chemical process catalysts, chemical instruments and methods, hydrogen and other directions, can solve the problems of high cost and poor activity of noble metal decoration, achieve mild conditions, improve hydrogen production performance, and prepare Simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation steps of the present invention are as follows:
[0018] Dissolve a certain amount of nickel sulfate hexahydrate and sodium selenite in a molar ratio of 1:1 in 40mL of ethylene glycol solvent, then add a certain amount of titanium dioxide (commercial P25), stir well and transfer to a high-pressure reactor , kept at 180°C for 24 hours, and finally cooled down to room temperature naturally. The obtained samples were centrifuged and washed with deionized water and absolute ethanol, and dried in a vacuum oven to obtain a series of NiSe / TiO with different NiSe loadings. 2 heterojunction photocatalysts. Accurately weigh 50 mg of the synthesized powder catalyst and place it in a photo-splitting water reactor to test the hydrogen production performance of photo-splitting water.
Embodiment 1
[0020] Dissolve a certain amount of nickel sulfate hexahydrate and sodium selenite (29.06 μmoL) in 40 mL of ethylene glycol solvent at a molar ratio of 1:1, then add a certain amount of titanium dioxide (40 mg, commercial P25), and stir well Afterwards, it was transferred to a high-pressure reactor, kept at 180°C for 24 hours, and finally cooled down to room temperature naturally. The obtained samples were washed by centrifugation with deionized water and absolute ethanol, and dried in a vacuum oven to obtain NiSe / TiO with a NiSe loading of 1%. 2 heterojunction photocatalysts. Accurately weigh 50 mg of the synthesized powder catalyst and place it in a photo-splitting water reactor to test the hydrogen production performance of photo-splitting water.
Embodiment 2
[0022] Dissolve a certain amount of nickel sulfate hexahydrate and sodium selenite (87.2 μmoL) in a molar ratio of 1:1 in 40 mL of ethylene glycol solvent, then add a certain amount of titanium dioxide (40 mg, commercial P25), and stir well Afterwards, it was transferred to a high-pressure reactor, kept at 180°C for 24 hours, and finally cooled down to room temperature naturally. The obtained samples were washed by centrifugation with deionized water and absolute ethanol, and dried in a vacuum oven to obtain NiSe / TiO with a NiSe loading of 3%. 2 heterojunction photocatalysts. Accurately weigh 50 mg of the synthesized powder catalyst and place it in a photo-splitting water reactor to test the hydrogen production performance of photo-splitting water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com