Patents
Literature
62results about How to "Improve hydrogen production activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
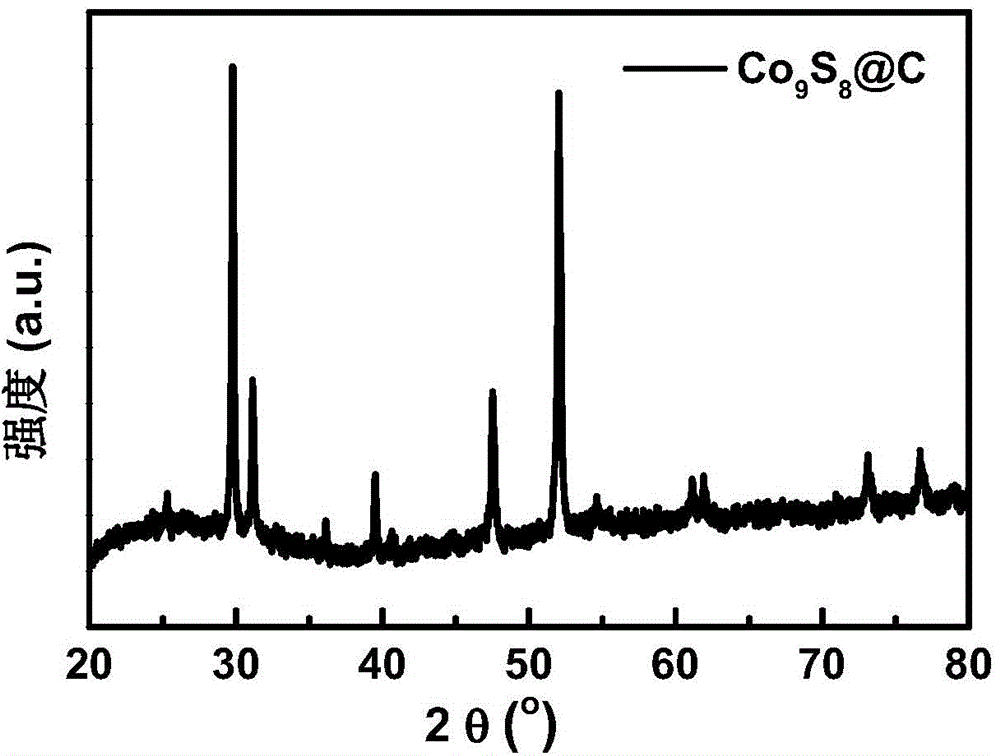
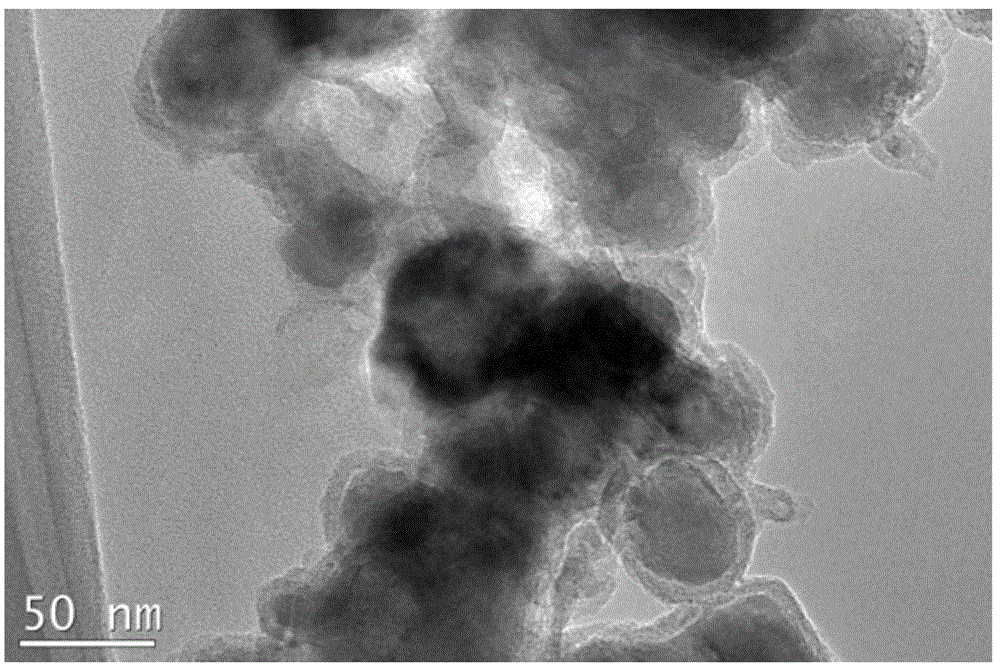
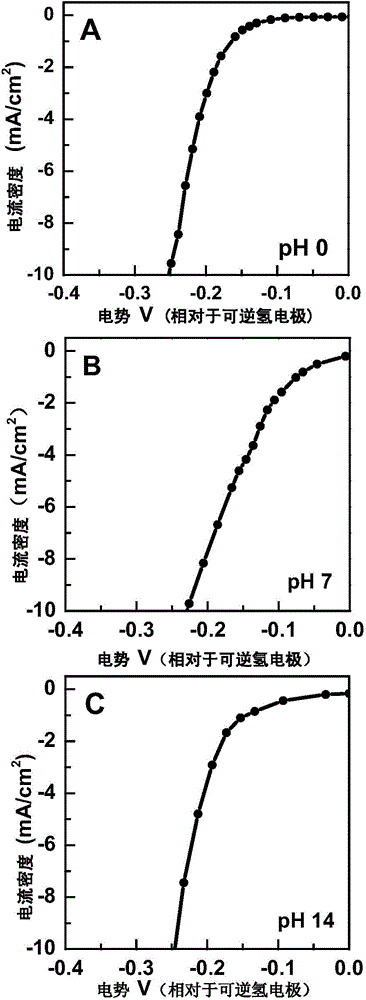
Carbon-coated cobalt sulfide material as well as preparing method thereof and application of carbon-coated cobalt sulfide material in aspect of water cracking hydrogen production
InactiveCN104399494AGood reproducibilitySynthetic raw materials are cheapElectrolysis componentsPhysical/chemical process catalystsCarbon coatingCobalt sulfide
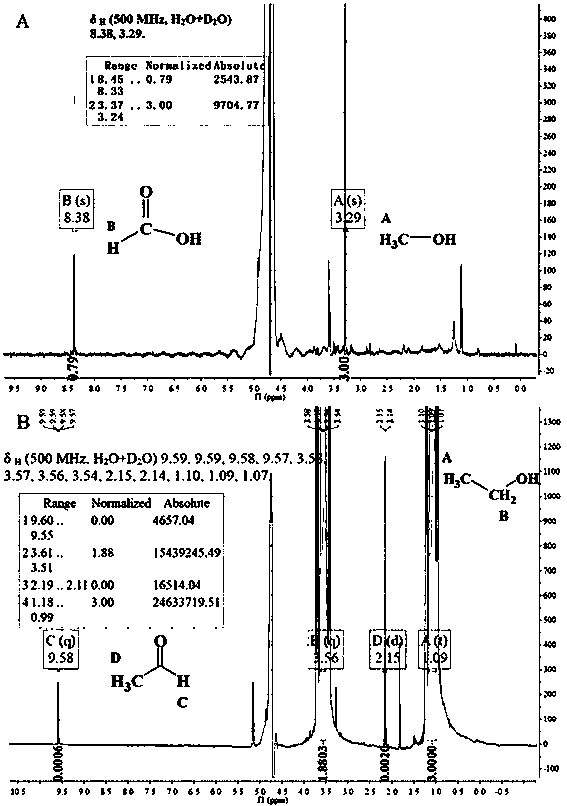
The invention provides a carbon-coated cobalt sulfide material as well as a preparing method thereof and application of the carbon-coated cobalt sulfide material as a catalyst in the aspect of hydrogen production through the electric catalytic cracking of water and belongs to the fields of catalyst synthesis technologies and application. An organic matter rich in sulfur is used as a sulfur source and a carbon source, a cobalt salt is used as a cobalt source, the organic matter and the cobalt salt are forged in an inert atmosphere after being mechanically mixed uniformly, and the carbon-coated cobalt sulfide material can be obtained. The preparing method is simple and controllable, adopts cheap raw materials, has good sample repeatability, spends short time in the synthesis process, has low requirements on equipment and is suitable for massive production. As an active substance is effectively protected by a carbon coating layer from electrolyte corrosion, the electric conductivity of the material is improved, and accordingly, the material shows excellent hydrogen production electric catalysis performance in acid, neutral as well as alkaline electrolytes. The most important is that the development of the catalyst capable of being used in the whole pH range opens a novel path for the preparation of a cheap, efficient and environment-friendly non-noble metal electric catalysis material for hydrogen production.
Owner:JILIN UNIV
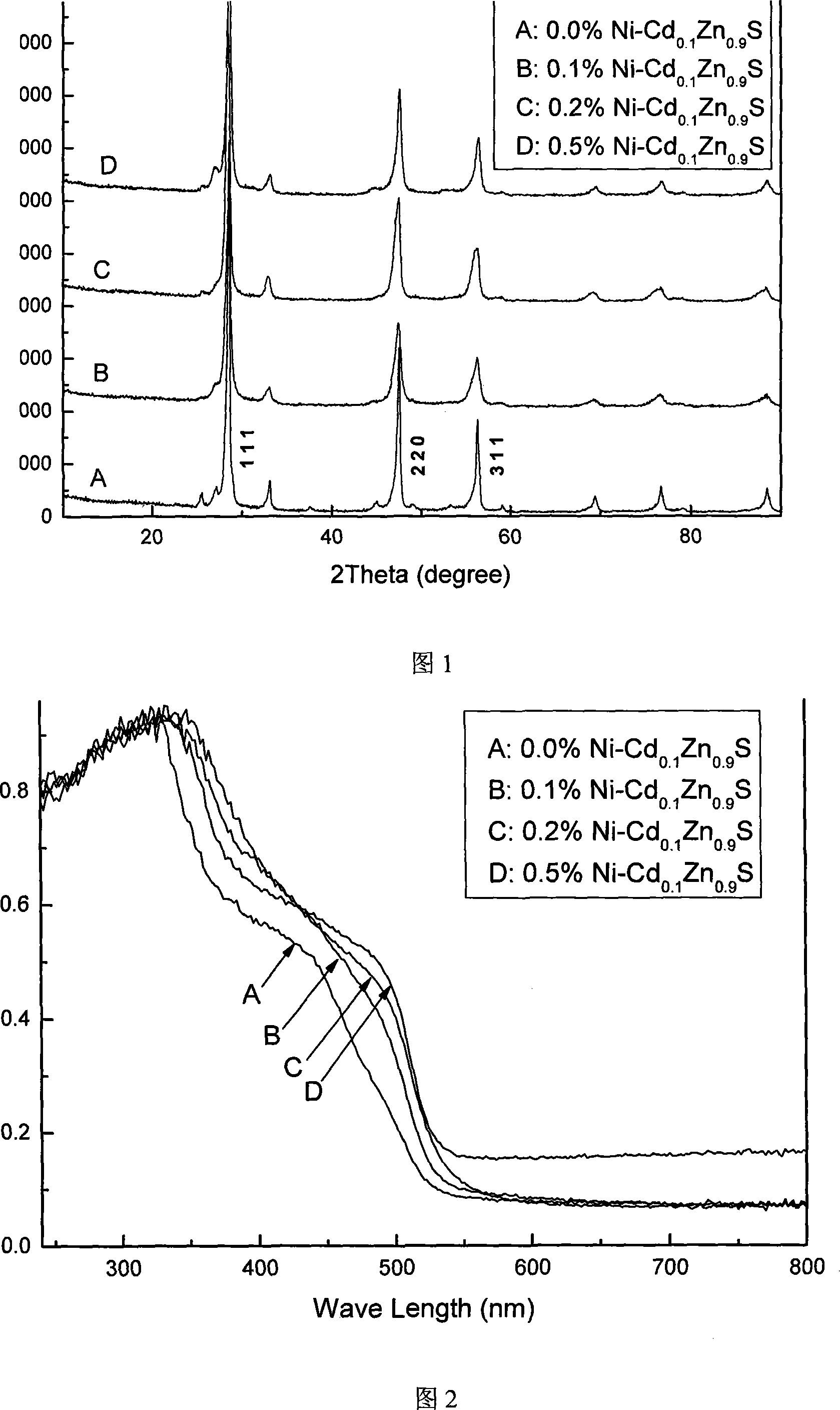
Ni doping Cd*.Zn*.*S micrometre ball photocatalyst and preparation method
ActiveCN101157044AImprove hydrogen production activityHigh activityPhysical/chemical process catalystsHydrogen productionPhotocatalytic water splittingPhotocatalytic reaction
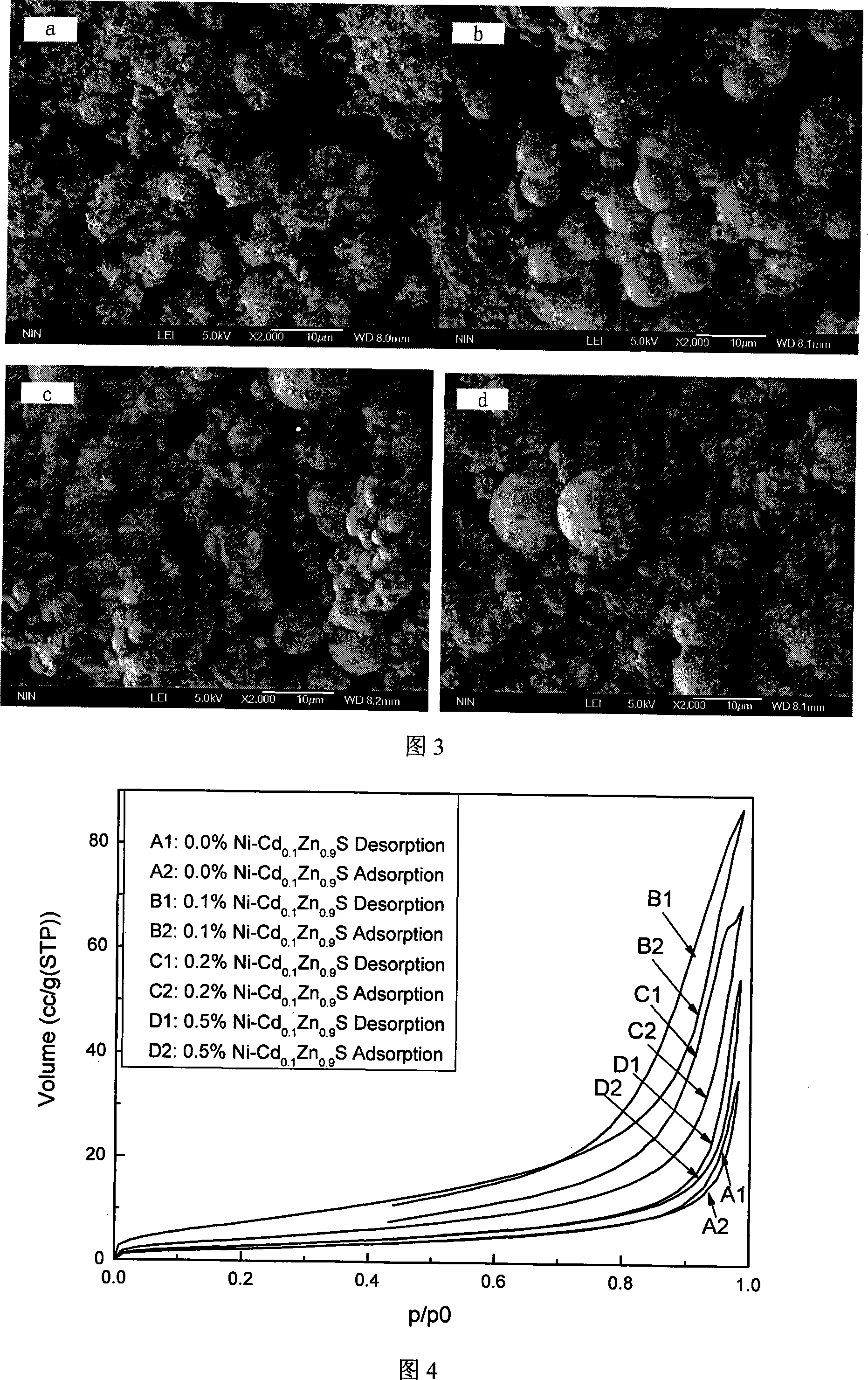
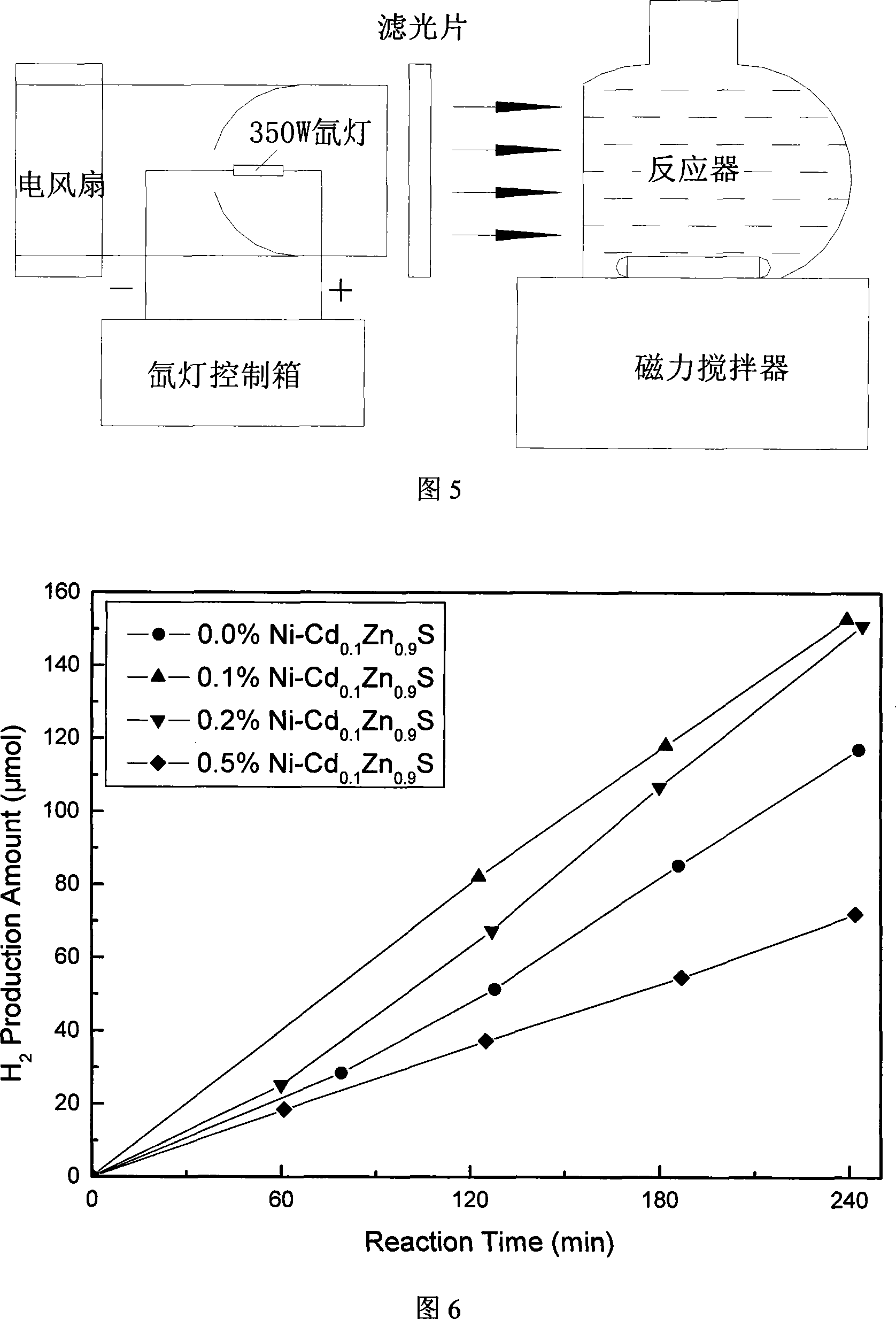
The invention discloses Ni-doped Cd0.1Zn0.9 S microsphere photocatalyst and the preparation method thereof, the particle shape of the produced photocatalyst is a microsphere formed by nanometer crystals, and the doping amount of Ni is 0.1 percent to 0.5 percent of the weight of the catalyst. The invention has the preparation method that zinc sulfate, cadmium sulfate, nickel nitrate, and thioacetamide are taken as raw materials to form mixed solution which is sealed in a hydrothermal caldron and then laid in a baking oven to be insulated, naturally cooled to the room temperature, a resultant is obtained through cleaning, drying, and grinding. The Ni-doped Cd0.1Zn0.9S microsphere photocatalyst is prepared by the invention, and the anti-surface oxidation capability in air and the photo-corrosion resistant capability in the photocatalytic reaction are evenly enhanced greatly. Because the particle of the photocatalyst is microsphere-shaped, the photocatalytic activity for photocatalytic water splitting into hydrogen of visible light of the catalyst is greatly enhanced, and the highest hydrogen production rate reaches 191.01 micromole / (g*h). The quantum efficiency reaches 6.77 percent at the 420 nm position. After Pt of 0.6 wt percent is loaded, the highest hydrogen production rate reaches 585.45 micromole / (g*h), which is three times than the hydrogen production rate when the Pt is not loaded. The quantum efficiency reaches 15.9 percent at the 420 nm position, the activity is high, and the stability is good.
Owner:XI AN JIAOTONG UNIV
Carbon dot/carbon nitride/titanium dioxide composite material as well as preparation method and application of the composite material
InactiveCN107837817AEnhance photocatalytic hydrogen production activityPromote migrationPhysical/chemical process catalystsHydrogen productionThree-phaseDioxide titanium
The invention discloses a carbon dot / carbon nitride / titanium dioxide composite material which is formed by compounding of three-phase carbon dots, graphene-like carbon nitride and titanium dioxide, wherein carbon nitride has a large surface area so as to just provide a depositing space for titanium dioxide nanosheets, so that titanium dioxide cannot cluster; and meanwhile, carbon nitride has relatively narrow energy gap and can increase the light response range. Furthermore, compounding of photo-induced electrons can be further inhibited by utilizing the unique electron transfer capacity of carbon dots, so as to increase the photocatalytic capacity. Moreover, the preparation method is mild in process conditions and low in cost, and the composite material is suitable for large-scale production, and has wide application prospect.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY
Preparation method of precious-metal-free high-activity photocatalytic-water-splitting hydrogen-producing catalyst
ActiveCN104324733AEnhance photocatalytic hydrogen production activityHigh activityPhysical/chemical process catalystsHydrogen productionPhotocatalytic water splittingHydrogen
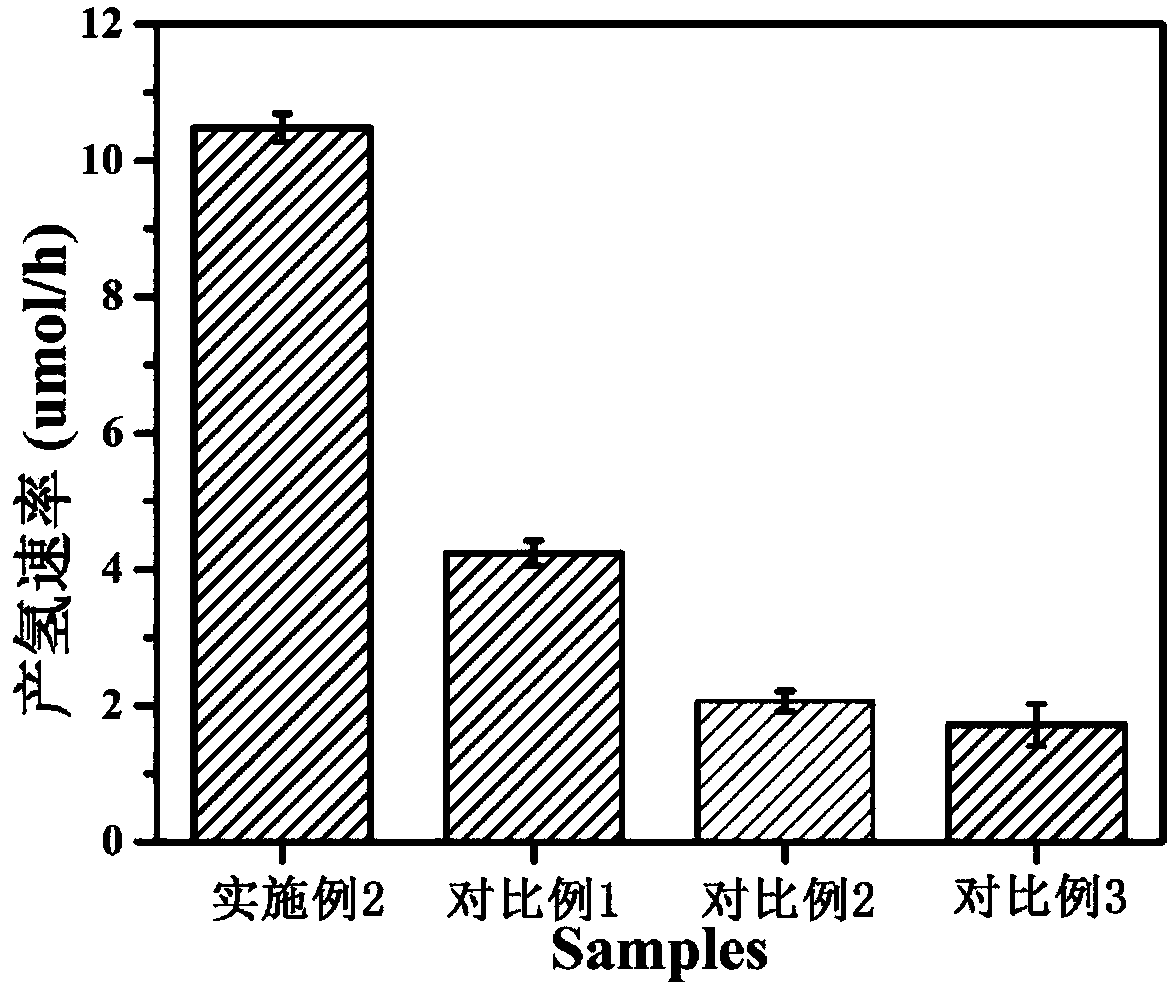
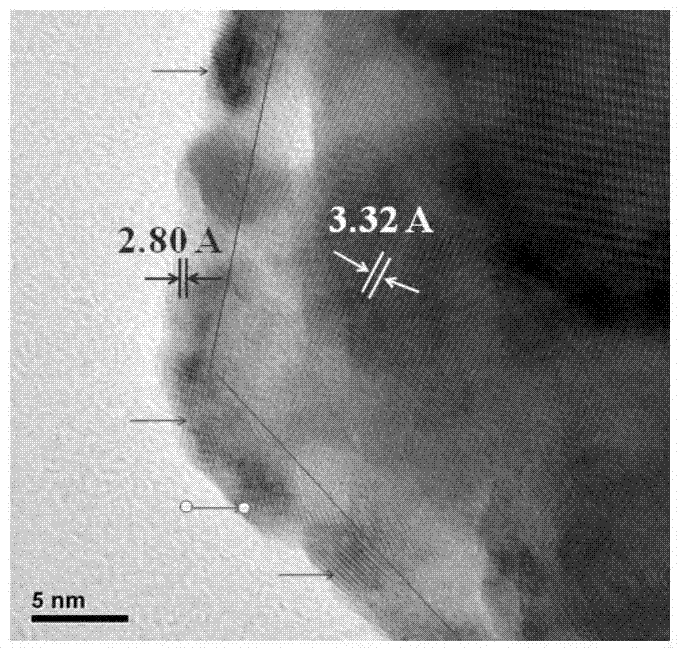
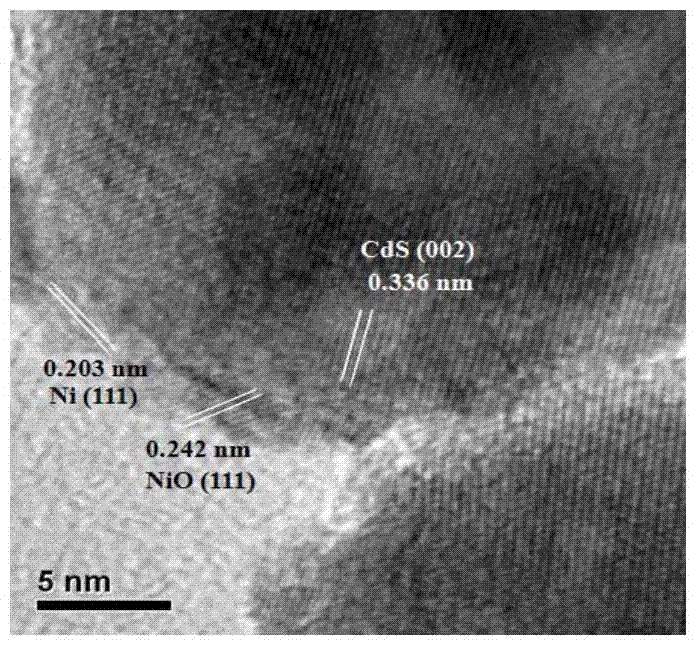
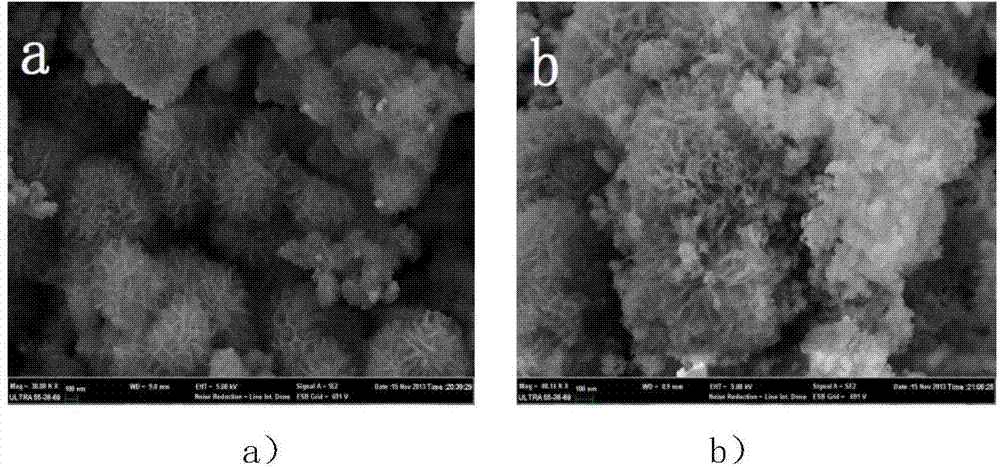
The invention discloses a preparation method of a precious-metal-free high-activity photocatalytic-water-splitting hydrogen-producing catalyst. In the invention, photocatalytically hydrogen-producing activities of a precious-metal-free NiOx-CdS photocatalyst, a precious-metal-free Ni-CdS photocatalyst and a precious-metal-free flower-shaped Ni / Ni(OH)2-CdS photocatalyst are all better than that of Pt-CdS, wherein the NiOx-CdS photocatalyst, the Ni-CdS photocatalyst and the Ni / Ni(OH)2-CdS photocatalyst are prepared respectively through an in-situ photodeposition method, a hydrothermal reduction method and a hydrothermal method. NiOx and Ni can effectively transfer and separate photoproduced electronics and holes so that the photocatalytically hydrogen-producing activity of the catalyst is enhanced. In addition, because of an unique flower-shaped structure of the flower-shaped Ni / Ni(OH)2, CdS can be inserted into a laminated structure of the Ni / Ni(OH)2 well and be contacted tightly with the Ni / Ni(OH)2, thereby forming a composite catalyst having an excellent photocatalytically hydrogen-producing activity under a photochemical effect.
Owner:SHANGHAI JIAO TONG UNIV
MoS2/TiO2NTs heterojunction photo-electro-catalyst substituting noble metal Pt sheet for hydrogen evolution and preparation method of MoS2/TiO2NTs heterojunction photo-electro-catalyst
InactiveCN106582721APromote adsorptionAdd reactive sitesCellsPhysical/chemical process catalystsSolventPhotocatalysis
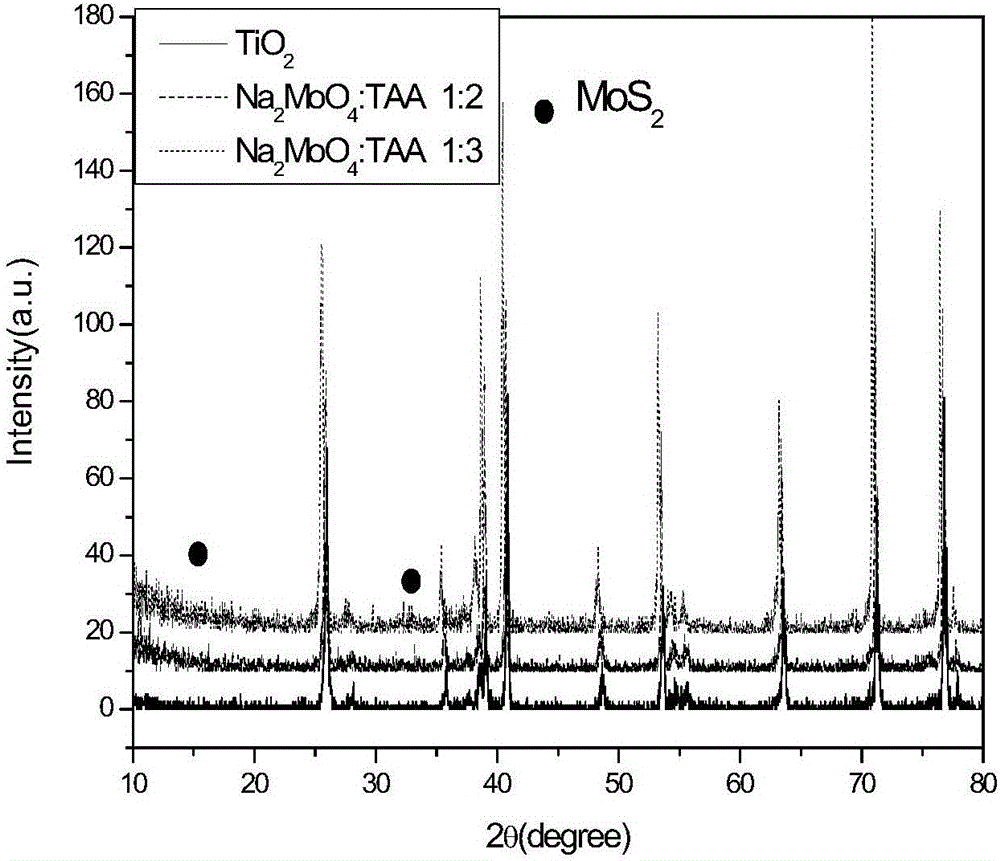
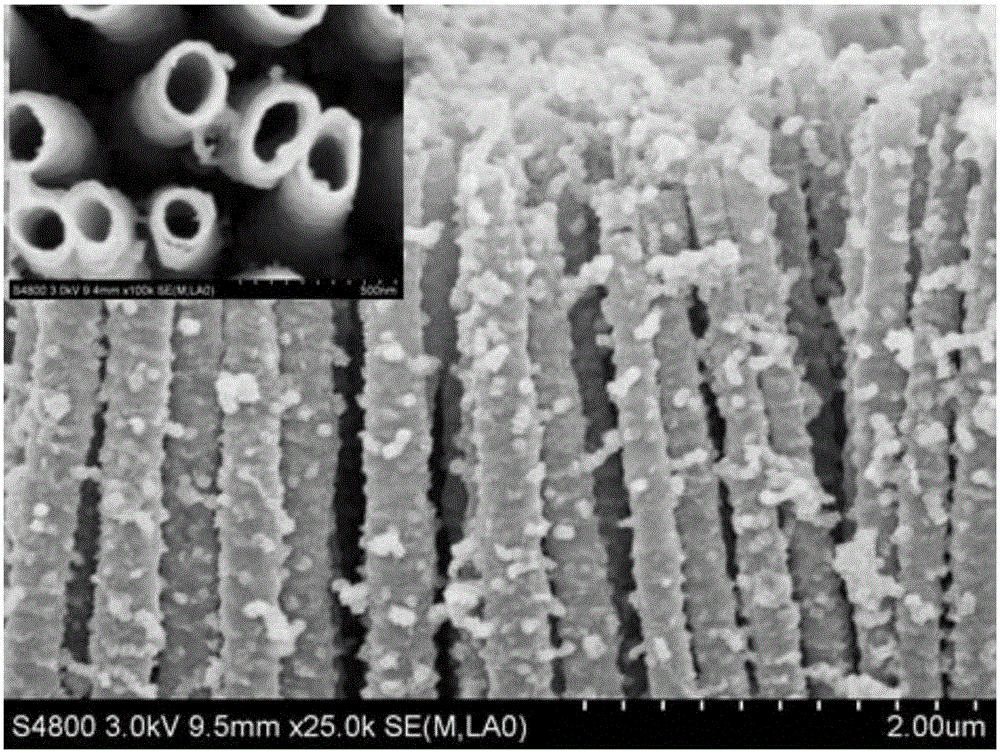
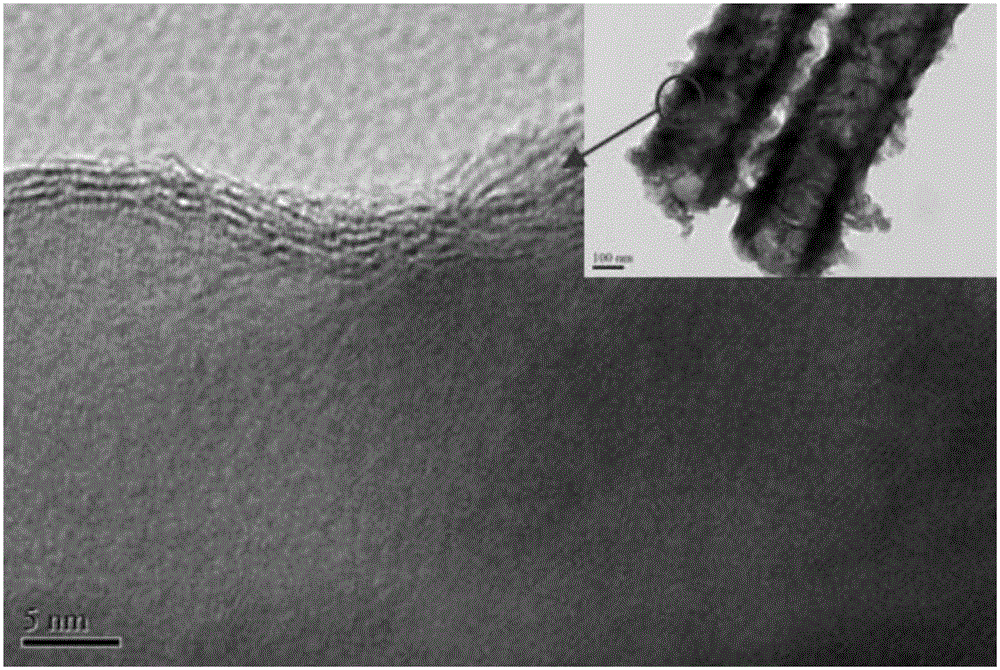
The invention discloses a MoS2 / TiO2NTs heterojunction photo-electro-catalyst substituting a noble metal Pt sheet for hydrogen evolution and a preparation method of the MoS2 / TiO2NTs heterojunction photo-electro-catalyst. The MoS2 / TiO2NTs heterojunction photo-electro-catalyst is synthesized by a two-step method. Firstly, a metal Ti sheet is subjected to anodic oxidation and TiO2NTs having a high specific surface area and gaps is formed by taking a diglycol and HF system as a solvent and the Ti sheet as a titanium source through an anodic oxidation process; secondly, MoS2 is loaded onto the prepared TiO2NTs by taking sodium molybdate and thiacetamide as a Mo source and a S source respectively, and taking diglycol as a solvent through kettle heating. A TiO2 surface layer structure, by using the specific surface area of a MoS2 layer, can improve the absorption to H2O molecules; in addition, MoS2 has excellent electron conduction ability and side belt effect, so that the number of reaction active sites are increased; photoelectrocatalysis collaboration can effectively separate electrons from cavities, so that the photocatalysis activity is improved, and hydrogen can be produced rapidly and efficiently by the decomposition in the presence of the MoS2 / TiO2NTs heterojunction photo-electro-catalyst.
Owner:SHANGHAI NORMAL UNIVERSITY
TiO2 photocatalyst for photocatalytic reforming biomass hydrogen preparation and preparation and application
InactiveCN101884914ASuppress generationHigh crystallinityHydrogenCatalyst activation/preparationHydrogenOrder of magnitude
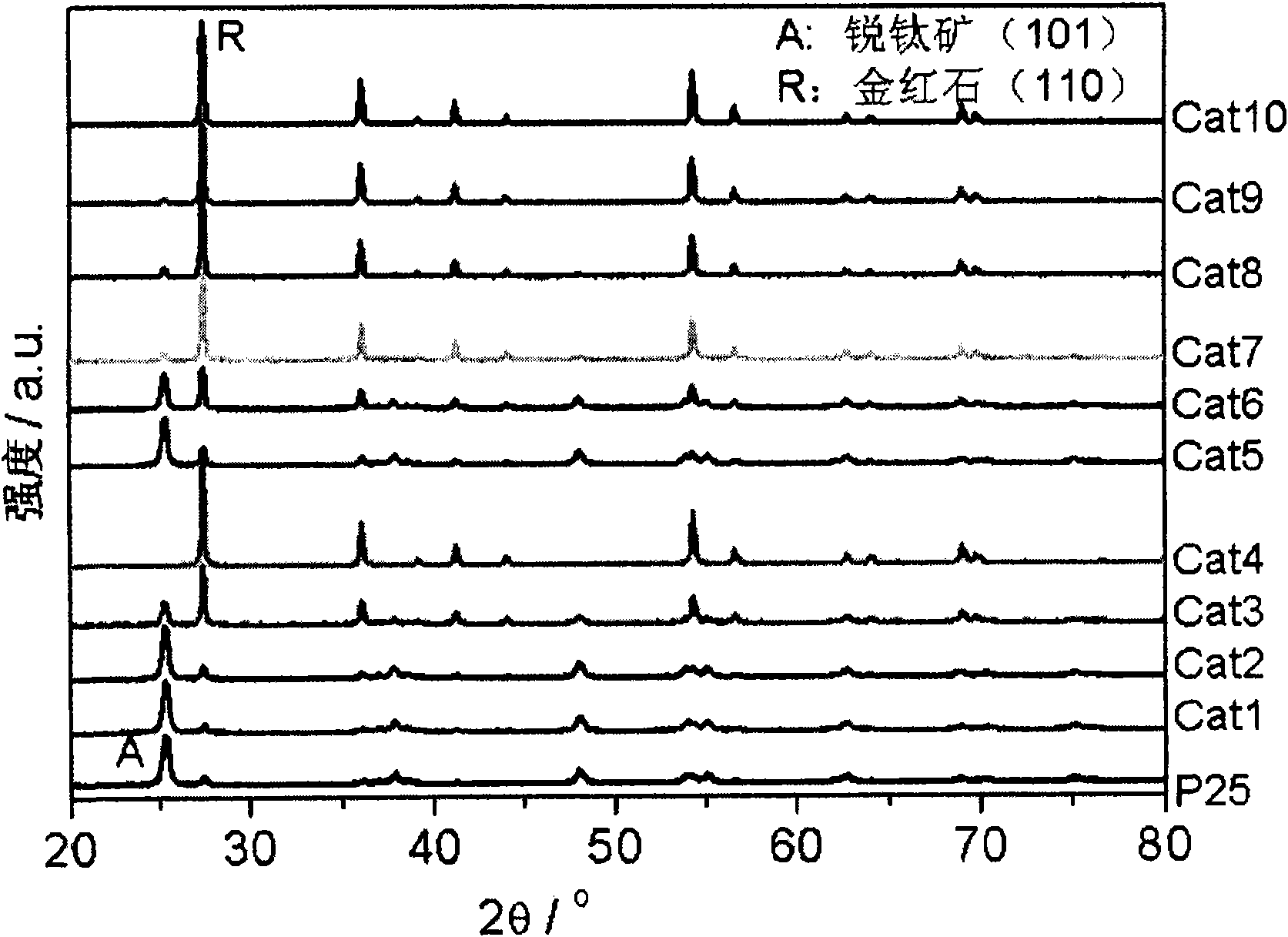
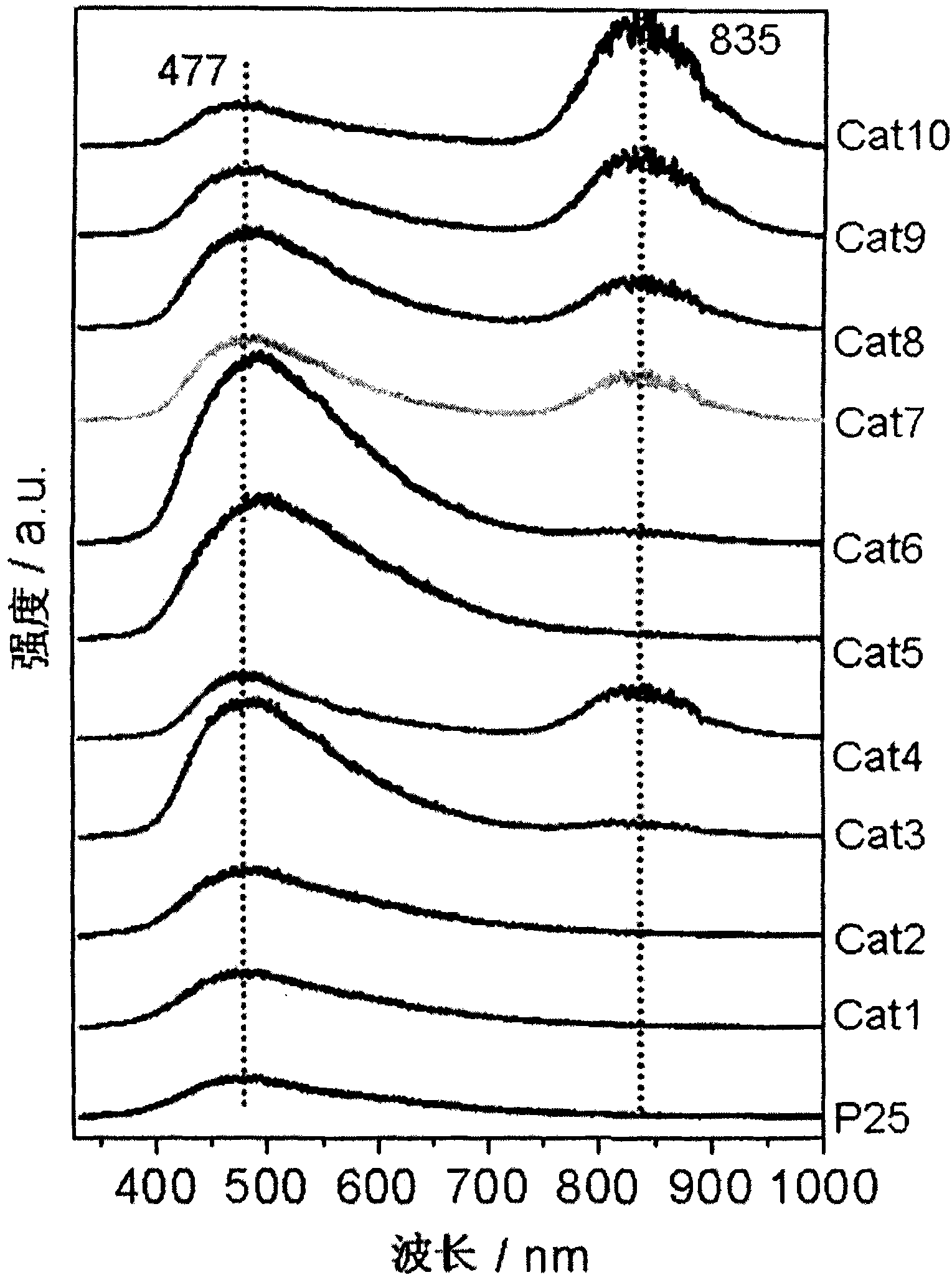
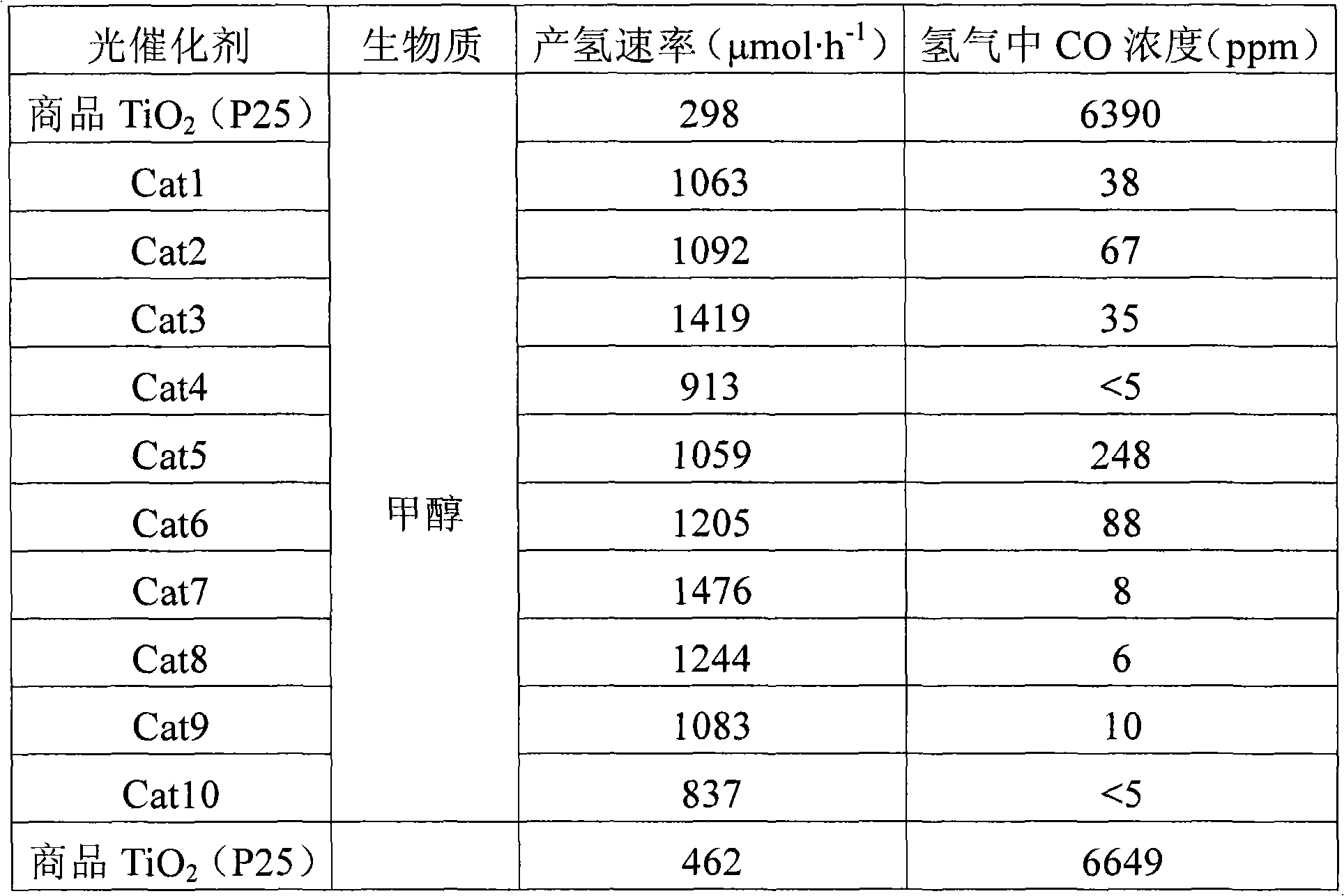
The invention discloses a TiO2 photocatalyst for photocatalytic reforming biomass hydrogen preparation. A crystal phase composition of anatase and rutile phases can be regulated in a wide range and is obtained by calculating the strengths of a stronger anatase diffraction peak (101) and a stronger rutile diffraction peak (110) on XRD. The TiO2 photocatalyst applied in the photocatalytic reforming biomass hydrogen preparation reaction greatly improves the hydrogen generation activity and effectively inhibits the generation of carbon monoxide, wherein in photocatalytic reforming methanol reaction, the hydrogen generation activity of the TiO2 photocatalyst is about five times that of a TiO2 reference agent (P25), and the CO content of the hydrogen is at least reduced by two orders of magnitude, even below 5ppm.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Two-dimensional nanosheet composite photocatalyst with bimetallic nanoparticles as heterojunction and preparation method thereof
InactiveCN106622322AEfficient use ofImprove hydrogen production activityCatalyst activation/preparationHydrogen productionHeterojunctionSchottky effect
The invention discloses a two-dimensional nanosheet composite photocatalyst with bimetallic nanoparticles as heterojunctions. The two-dimensional nanosheet composite photocatalyst is composed of a photocatalytic active component and the bimetallic nanoparticles uniformly distributed on the photocatalytic active component by virtue of a binding agent, wherein the photocatalytic active component is a two-dimensional g-C3N4 nanosheet, the binding agent is 0.1-5wt% Nafion perfluorinated resin solution, and the bimetallic nanoparticles are Pd-Ag bimetallic nanoparticles. Raw materials used by the composite photocatalyst are environment-friendly materials respectively, and the Pd-Ag bimetallic nanoparticle heterojunctions have surface plasma resonance effect and interface schottky effect and can more effectively utilize visible light and inhibit recombination of photo-induced electrons and holes, so that the composite photocatalyst disclosed by the invention has high activity and good stability when being applied to hydrogen production through photocatalytic decomposition of water.
Owner:HENAN POLYTECHNIC UNIV
Preparation method of porous carbon nitride photocatalyst
ActiveCN109046420AThe preparation process is simple and easy to operateEnhanced Photocurrent Response CapabilityPhysical/chemical process catalystsHydrogen productionTube furnaceDecomposition
The invention belongs to the technical field of photocatalytic material preparation, and relates to a preparation method of a porous carbon nitride photocatalyst. The method concretely comprises the following steps: (1) putting melamine in a ceramic crucible and heating to a certain temperature in a tube furnace to obtain a carbon nitride precursor; (2) collecting the obtained carbon nitride precursor, grinding, and then weighing a certain amount to be horizontally paved in a ceramic ark; and (3) putting the ark, which is loaded with the carbon nitride precursor, into the tube furnace, and calcining in a pure oxygen atmosphere to obtain a porous carbon nitride material. The method has the advantages that the preparation process is simple and easy to operate, and any template agents and corrosive chemical reagents such as strong acid and strong base are not adopted, thereby being green and environment-friendly; the prepared porous carbon nitride photocatalyst has an enlarged specific surface area and shows good photocatalytic water decomposition hydrogen generating performance and light current responsiveness, and the used raw materials are cheap, easy to obtain and convenient for volume production.
Owner:JIANGSU UNIV
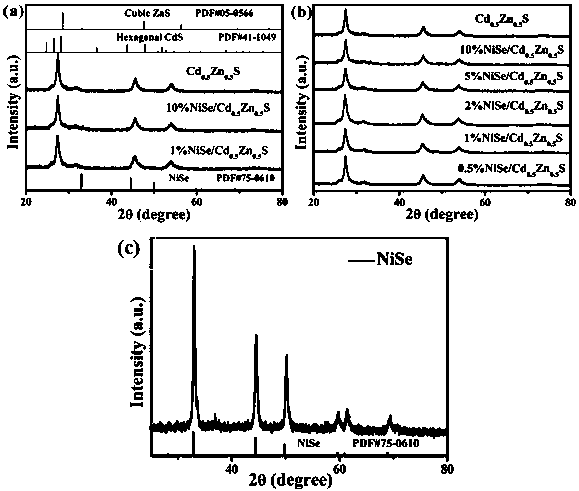
Efficient heterojunction photocatalyst taking NiSe as aid as well as preparation method and application thereof
InactiveCN110075875ASimple thermal reactionEasy to makePhysical/chemical process catalystsHydrogen productionHeterojunctionNickel sulfate hydrate
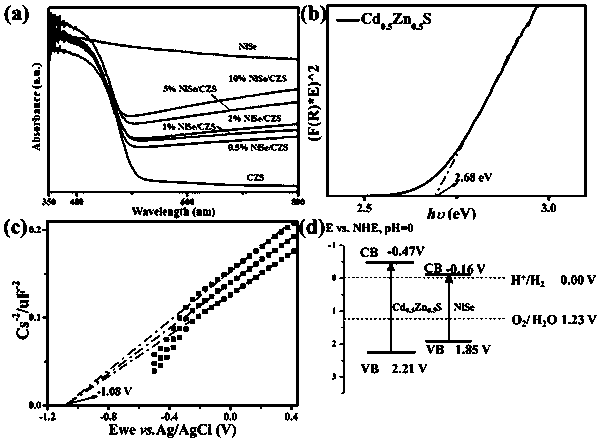
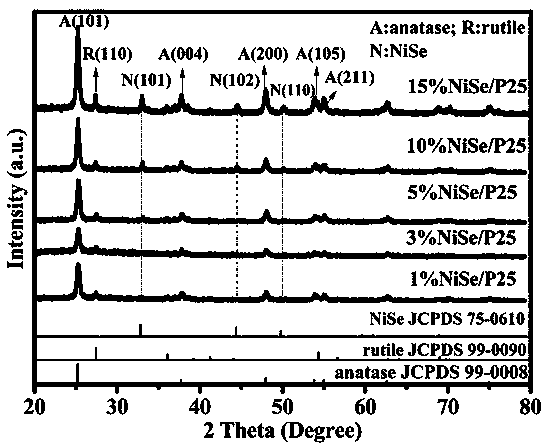
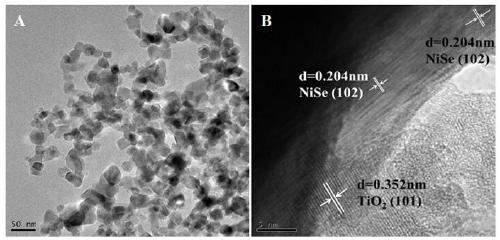
The invention discloses a visible light driven NiSe-based heterojunction photocatalyst for performing efficient photocatalysis on water to perform pyrolysis to produce hydrogen. The heterojunction photocatalyst is prepared by the following steps: preparing Cd0.5Zn0.5S sosoloid by taking cadmium acetate, zinc acetate and thioacetamide as cadmium, zinc and sulfur sources correspondingly and adoptinga precipitation-hydrothermal method; and taking nickel sulfate hexahydrate, sodium selenite and the synthesized Cd0.5Zn0.5S sosoloid as substrates, taking glycol as a solvent and a reducing agent, and synthesizing a NiSe / Cd0.5Zn0.5S heterojunction photocatalyst through a one-step solvent-thermal method. The maximum hydrogen production rate of the heterojunction photocatalyst under visible light reaches 70.3 mmol / h / g, and the activity of the heterojunction photocatalyst is 2.35 times that of pure Cd0.5Zn0.5S sosoloid; furthermore, the preparation method is green, environmentally-friendly, simple to operate and stable in activity, the utilization rate of sunlight, particularly visible light by the photocatalyst is greatly increased, and higher economic benefit can be achieved.
Owner:FUZHOU UNIV
Preparation method and application of nitrogen defect/boron doped tubular carbon nitride photocatalyst
InactiveCN113318764AIncrease exposurePromote absorptionWater/sewage treatment by irradiationWater treatment compoundsPtru catalystPhosphoric acid
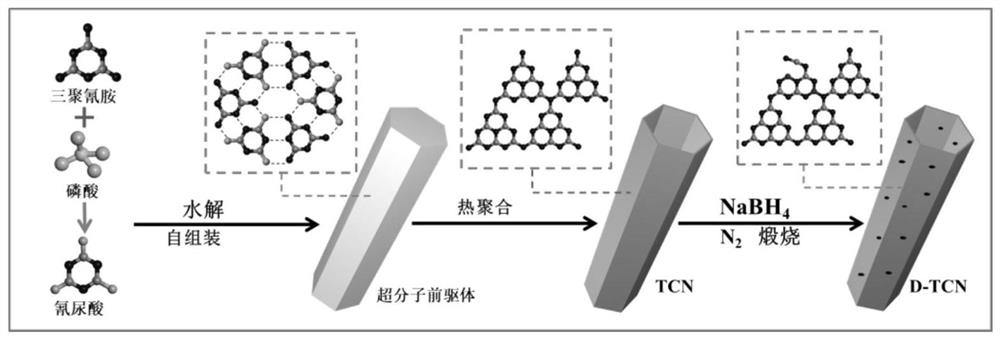
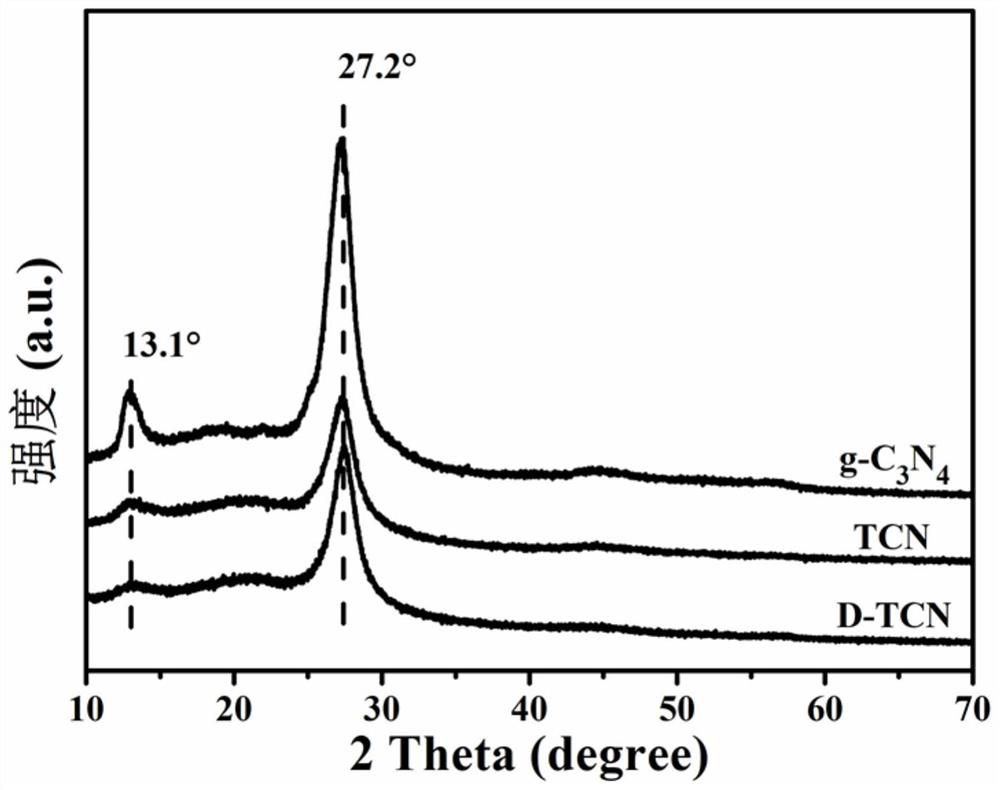
The invention belongs to the technical field of semiconductor photocatalysts, and particularly relates to a preparation method and application of a nitrogen defect / boron doped tubular carbon nitride photocatalyst. The preparation method comprises the following steps: putting melamine and phosphoric acid into a high-pressure kettle containing deionized water to carry out hydrothermal reaction, washing the reacted product with deionized water, carrying out vacuum drying to obtain a substance which is a supramolecular precursor, conducting calcining again, naturally conducting cooling and conducting grinding to obtain tubular carbon nitride; mixing tubular carbon nitride and NaBH4, then conducting uniform grinding, and putting the mixture into a porcelain boat for calcination; and washing the calcined product with HCl and distilled water, and carrying out vacuum drying to obtain the nitrogen defect / boron doped tubular carbon nitride photocatalyst, which is marked as a D-TCN photocatalyst. The synergistic effect of nitrogen defect / boron doping and the tubular structure can effectively promote light capture, accelerate charge transfer and promote exposure of more active sites, and the effects of photocatalytic degradation of antibiotics and decomposition of water to produce hydrogen activity can be remarkably enhanced.
Owner:JIANGSU UNIV
Preparation method and aplication of supported photocatalyst NiSe2/CdS
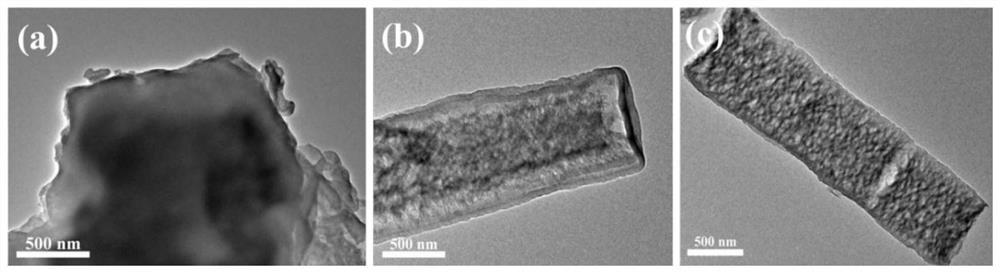
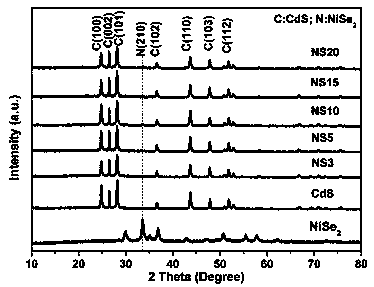
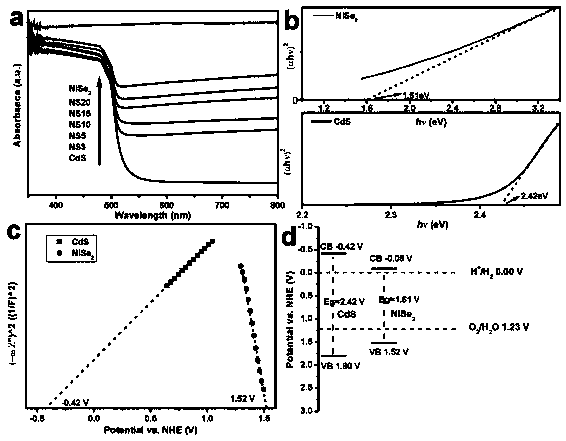
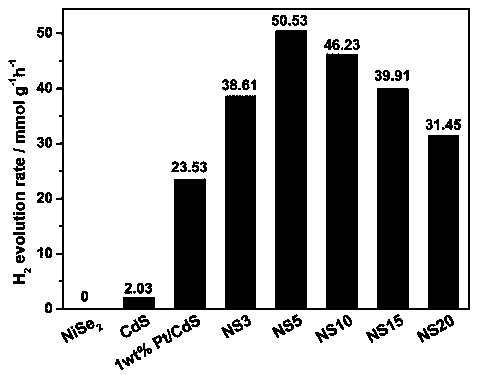
ActiveCN110280276AEasy to makeMild conditionsCatalyst activation/preparationHydrogen productionHeterojunctionPhotocatalytic water splitting
The invention belongs to the field of preparation and application of photocatalysts, and relates to a visible-light-driven NiSe2-based heterojunction photocatalyst for producing hydrogen through efficient photocatalytic water splitting. The NiSe2 / CdS heterojunction photocatalyst is synthesized through a second-step solvothermal method by firstly preparing CdS nano rods by taking cadmium nitrate and thiourea as a cadmium source and a sulfur source respectively and adopting a solvothermal method, then performing a hydrothermal reaction by taking nickel nitrate hexahydrate, hexamethylenetetramine, trisodium citrate and the synthesized CdS nano rods as substrates and taking deionized water as a solvent, then cooling, then adding an NaHSe solution, and then continuing to raise the temperature for reacting. The heterojunction photocatalyst involved in the invention has high hydrogen production rate in visible light; a preparation method is environmentally friendly; the operation is simple; the activity is stable; the utilization rate of the photocatalyst to sunlight, especially the visible light, is greatly improved; higher economic benefit can be obtained.
Owner:FUZHOU UNIV
Preparation method of phosphorus-doped cadmium sulfide supported nickel carbide quantum-dot nanorod photocatalyst
ActiveCN109731588ASimple microscopic morphologyRegular and unique microscopic morphologyPhysical/chemical process catalystsN dimethylformamideRoom temperature
The invention discloses a preparation method of a phosphorus-doped cadmium sulfide supported nickel carbide quantum-dot nanorod photocatalyst. The preparation method includes the steps of 1) mixing CdS nanorods and NaH2PO2, heating the mixture in N2 atmosphere to obtain phosphorus-doped CdS nanorods; 2) dissolving the phosphorus-doped CdS nanorods in N,N-dimethylformamide, adding Ni(NO3)2 6H2O and2-methylimidazole, and stirring at room temperature to obtain a precursor material; 3) heating the precursor material of the step 2), holding the temperature, cooling to room temperature, washing with deionized water and ethanol to obtain a black-green product, and drying the black-green product to obtain the phosphorus-doped cadmium sulfide supported nickel carbide quantum-dot nanorod photocatalyst. The photocatalyst prepared via the preparation method has good stability and high visible light catalytic activity, and the preparation method is simple.
Owner:XI AN JIAOTONG UNIV
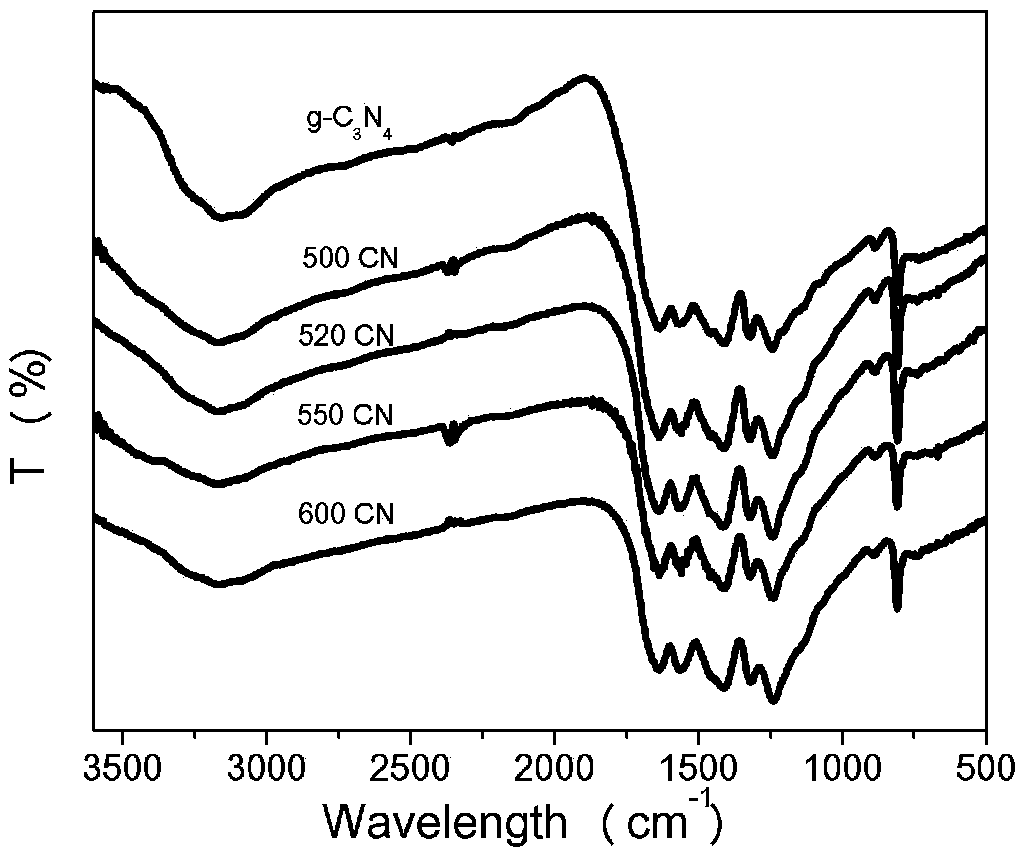
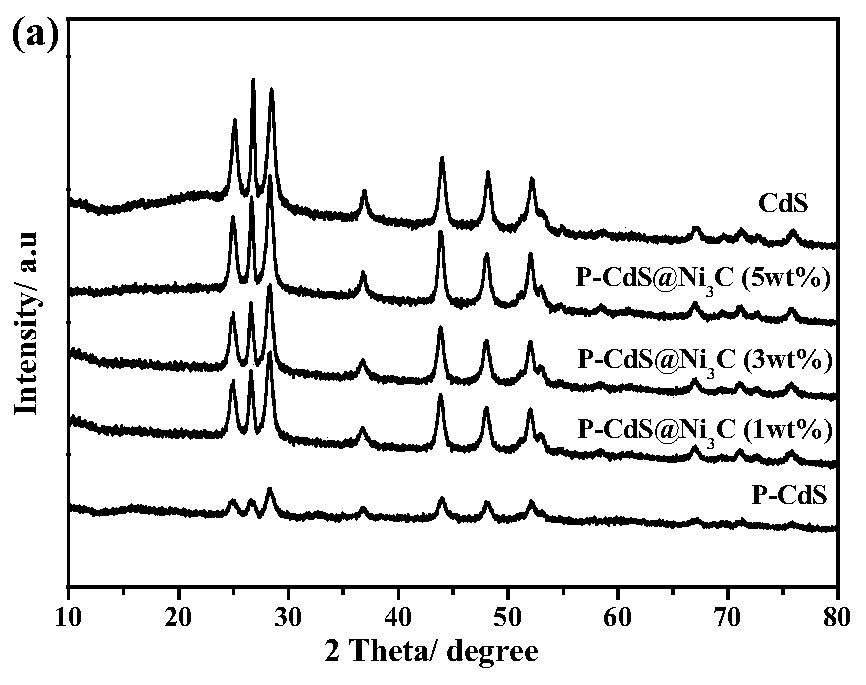
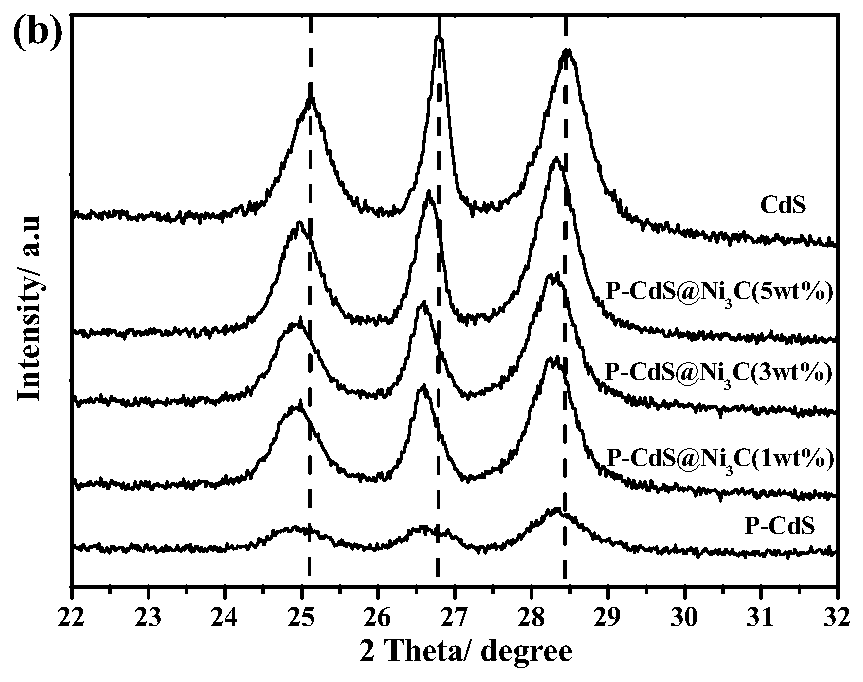
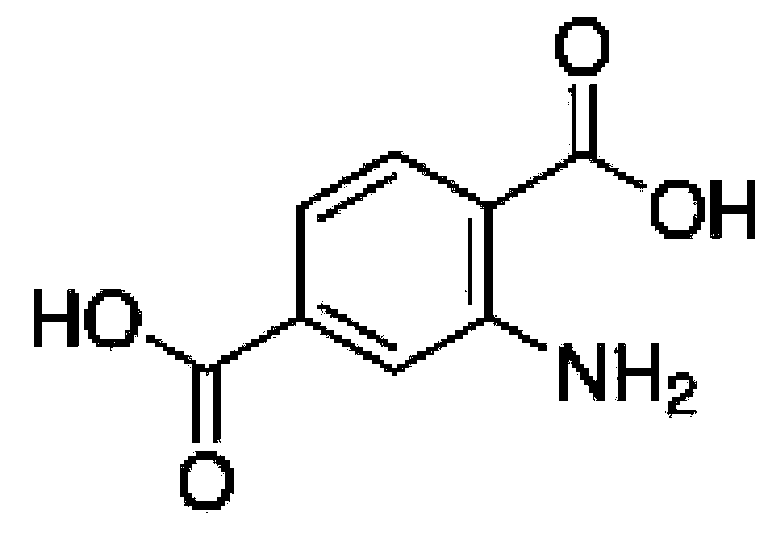
Method for preparing graphite-phase carbon nitride visible-light-induced photocatalyst through copolymerization of 2-aminoterephthalic acid and amine compound
ActiveCN109876841AOptimize chemical compositionImprove separation efficiencyCatalyst activation/preparationHydrogen productionCarbon nitrideGraphite
The invention relates to a method for preparing a graphite-phase carbon nitride visible-light-induced photocatalyst by copolymerization of 2-aminoterephthalic acid and an amine compound. The method comprises the following steps: uniformly mixing the amine compound and the 2-aminoterephthalic acid to obtain mixed powder, and calcining the mixed powder at 520-600 DEG C to obtain the graphite-phase carbon nitride visible-light-induced photocatalyst. The preparation method is simple, and the obtained novel graphite-phase carbon nitride has better visible light catalytic hydrogen production performance compared with original carbon nitride.
Owner:XI AN JIAOTONG UNIV
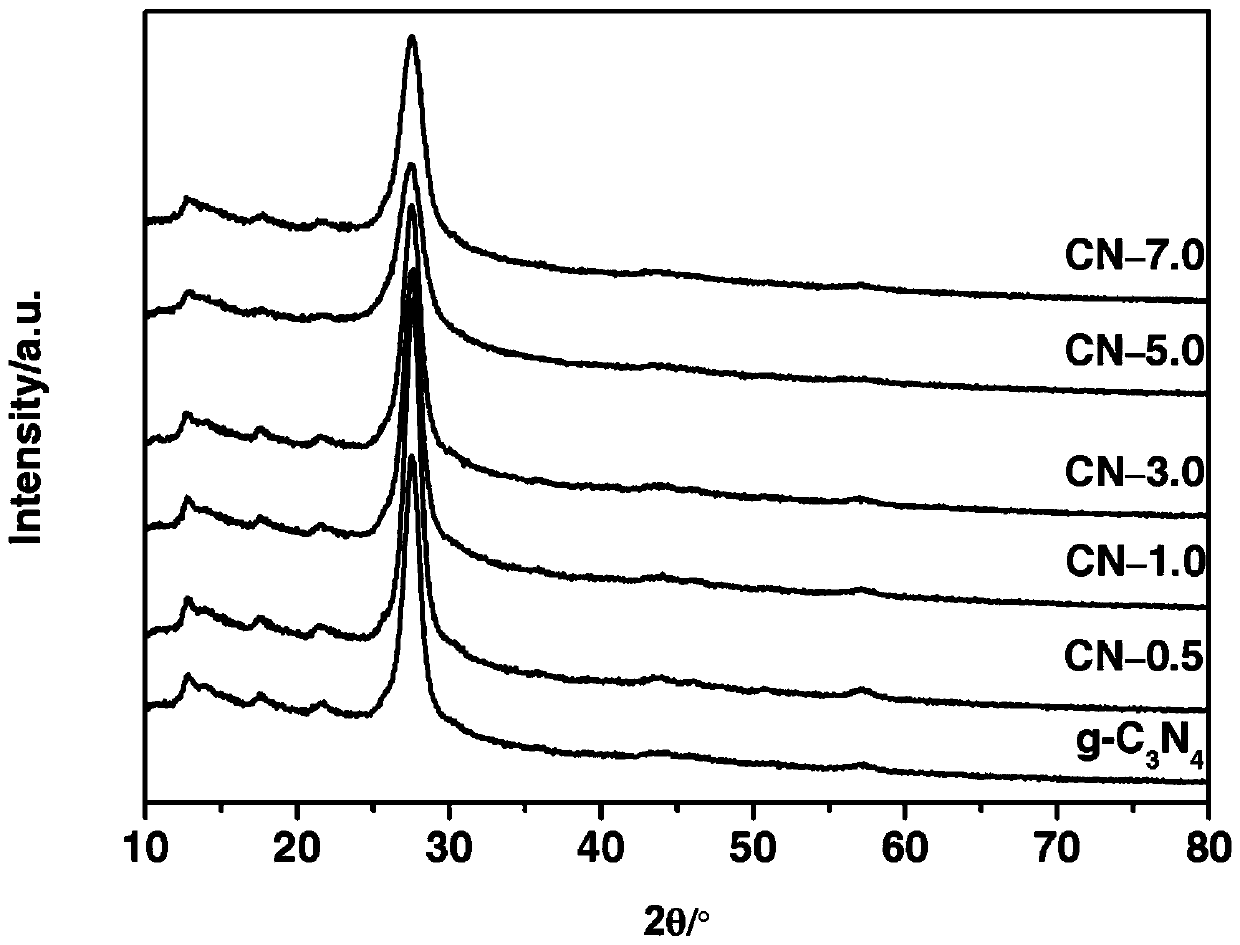
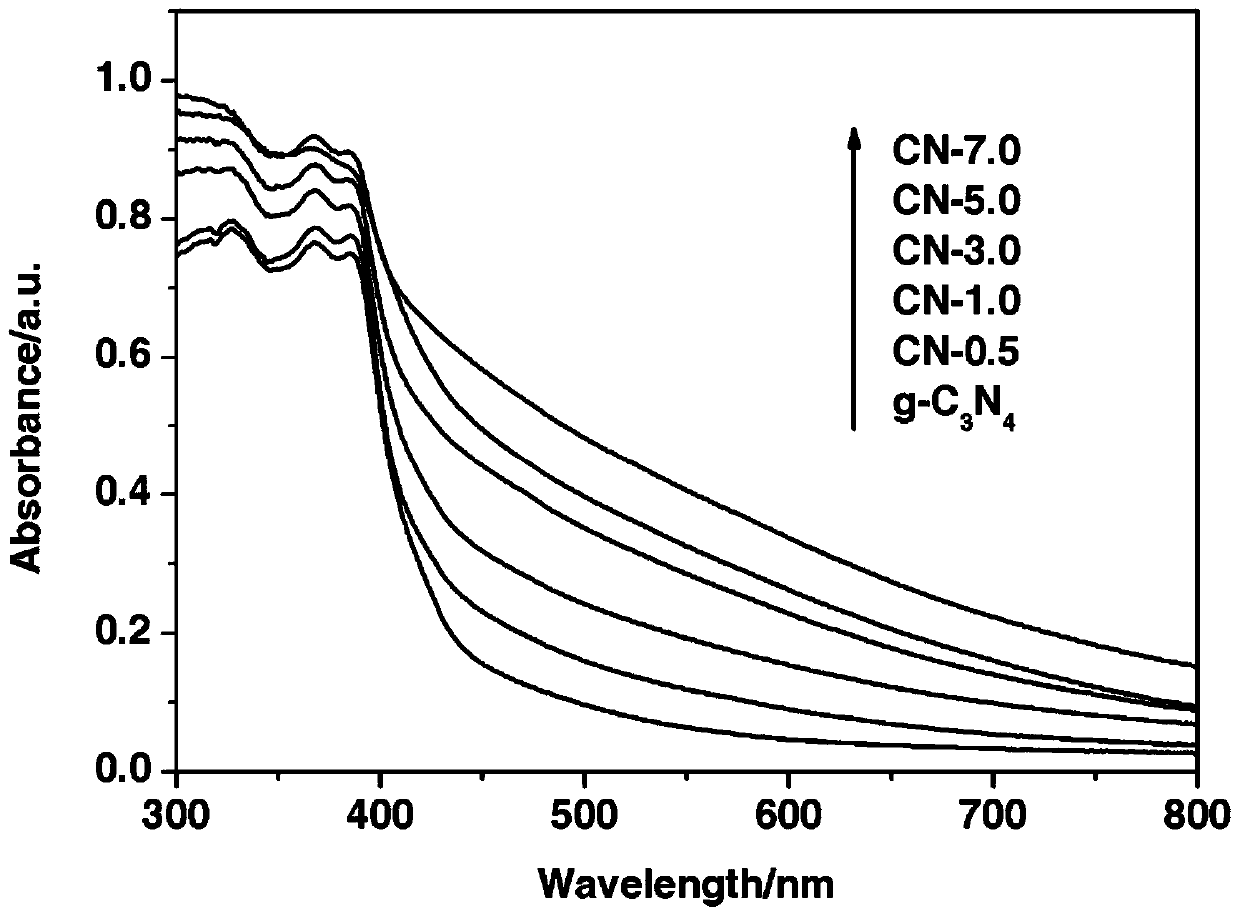
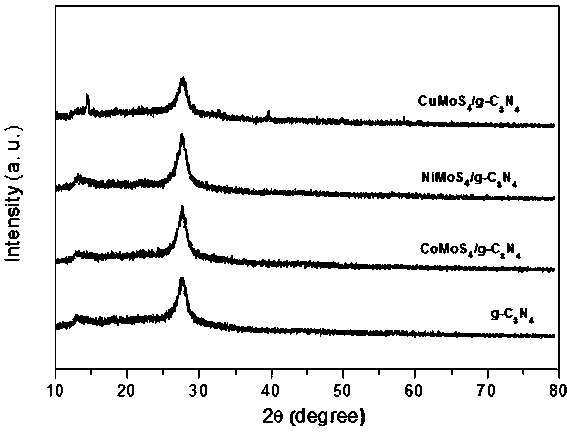
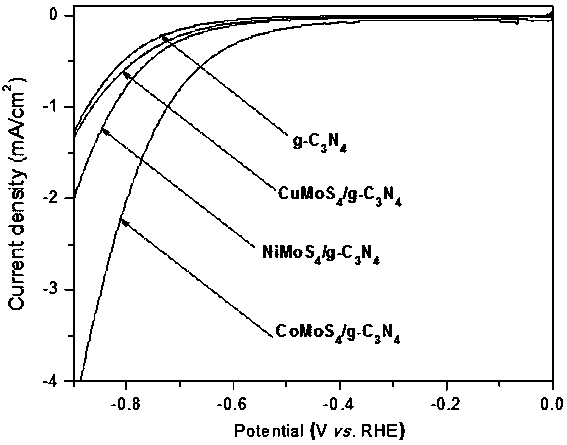
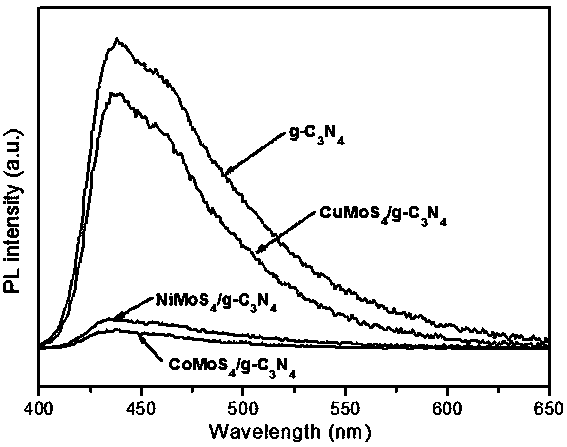
Ternary metal sulfide/graphite phase carbon nitride composite photocatalytic material, and preparation method and application thereof
InactiveCN107930671AHigh activityImprove hydrogen production activityPhysical/chemical process catalystsHydrogen productionMaterials preparationAlcohol
The invention belongs to the technical field of material preparation and photocatalysis, and particularly relates to an MMoS4 / g-C3N4 (M is equal to Co, Ni, Cu) composite photocatalytic material, and apreparation method and application thereof. MMoS4 particles are loaded on graphite phase carbon nitride through a coprecipitation method so as to form the composite photocatalytic material; the preparation method has the advantages of simple and convenient preparation process, low coat, and easy large-scale industrial production; the prepared composite photocatalytic material has a heterojunctionstructure formed by compounding ternary metal sulfide and g-C3N4, so that the separation of a photon-generated carrier is greatly promoted, a surface hydrogen production dynamic behavior is improved,the hydrogen production activity of a photocatalyst is remarkably improved, and the photocatalytic material has favorable environmental stability and can be applied to visible light photocatalysis hydrogen production in an alcohol-water system.
Owner:FUZHOU UNIV
Anthemia-shaped NiS/ZnS visible light photocatalyst and preparation method thereof
InactiveCN104399493AWide accessEasy to industrializePhysical/chemical process catalystsEthylenediamineNickel salt
The invention discloses an anthemia-shaped NiS / ZnS visible light photocatalyst and a preparation method thereof, the anthemia-shaped NiS / ZnS visible light photocatalyst comprises the following raw materials by weight: 1-5 parts of an inorganic zinc salt, 2-10 parts of thioacetamide or thiourea, 38-50 parts of ethanolamine or ethylenediamine, and 0.01-0.02 part of an inorganic nickel salt. The method is as follows: adding the inorganic zinc salt and thioacetamide or thiourea into a container filled with the ethanolamine or ethylenediamine, stirring evenly at less than 45 DEG C, standing for 15-20min to obtain suspension; reacting in microwave at the temperature of 140-150 DEG C for 4-5min, cooling a reaction kettle to room temperature to obtain an anthemia-shaped ZnS complex precursor; reacting in microwave at the temperature of 140-150 DEG C for 20-25min to obtain a primary product, centrifugally drying to obtain the anthemia-shaped NiS / ZnS visible light photocatalyst. The anthemia-shaped NiS / ZnS visible light photocatalyst has the advantages of simple method operation, low cost and wide raw materials, can be recycled, has excellent visible light photocatalytic hydrogen production activity under visible light irradiation, and can promote photocatalytic hydrogen production industrialization.
Owner:武汉钢铁有限公司
Heterojunction photocatalyst for hydrogen production and alcohol oxidation by high-efficiency photocatalytic water-cracking
ActiveCN108993546AImproving the hydrogen production performance of photocatalytic water splittingEasy to makePhysical/chemical process catalystsHydrogen productionHeterojunctionLight energy
The invention discloses a heterojunction photocatalyst for hydrogen production and alcohol oxidation by high-efficiency photocatalytic water-cracking, and belongs to the field of photocatalyst preparation and application. According to the invention, nickel sulfate hexahydrate, sodium selenite and titanium dioxide are taken as reactants, glycol is taken as a solvent and a reducing agent, and then aNiSe / TiO2 heterojunction photocatalyst is synthesized according to a one-step solvothermal method. The heterojunction photocatalyst prepared by the invention has a photocatalytic hydrogen productionperformance 9 times higher than that of pure TiO2, and shows good stability in the long-cycle operation. Furthermore, simple alcohols are taken as sacrificial agents, and chemicals of high-value fuel(hydrogen), small molecular acids, aldehydes and the like are prepared through the photocatalytic water cracking under illumination of simulated sunlight. In addition, the heterojunction photocatalystused in the invention is green and simple in preparation method, rich in material sources, low in cost and stable in activity, and combines the small molecular alcohols as the sacrificial agents, sothat economic benefits of photocatalysis and the utilization rate of absorbed light energy can be greatly improved.
Owner:FUZHOU UNIV
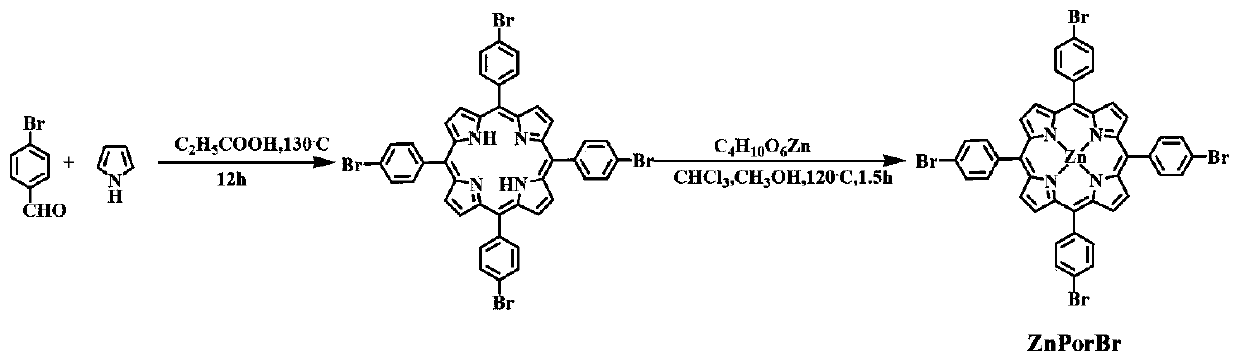
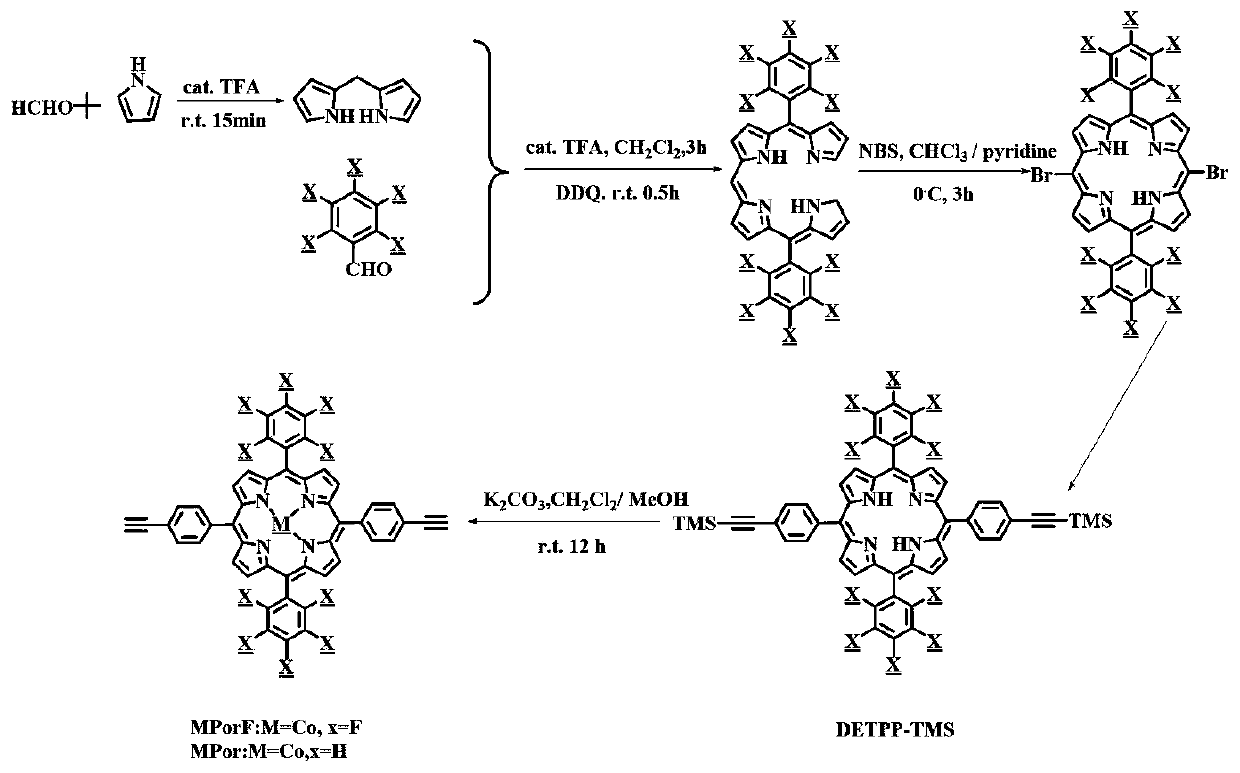
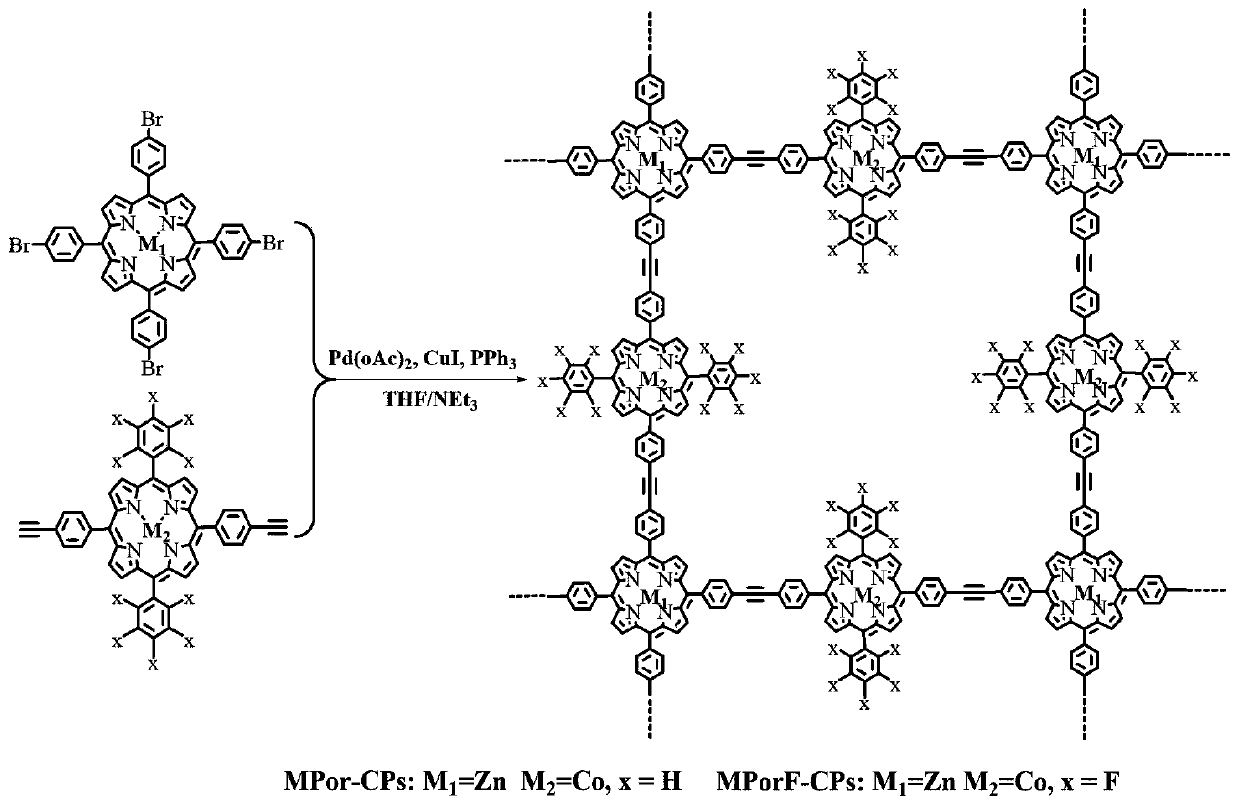
Fluorine-substituted Zn/Co porphyrin-based conjugated organic polymer as well as preparation method thereof and application thereof
ActiveCN110951050AEnhanced light absorptionEnhance photocatalytic hydrogen production activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionSpectral responsePorphyrin
The invention discloses a fluorine-substituted Zn / Co porphyrin-based conjugated organic polymer as well as a preparation method thereof and application thereof. Required raw materials are wide in source, the synthesis process is easy to control, a Sonogashira coupling reaction is adopted to expand the light absorption range of a porphyrin complex to a near-infrared light region, and the transfer of photo-induced electrons between Zn and Co center metals can be accelerated, and the recombination of the electrons and holes is reduced, so that photons in an ultraviolet-visible-near-infrared lightregion are effectively utilized to realize efficient photocatalytic hydrogen production, and a good application prospect is shown in the aspect of photocatalytic hydrogen production with wide spectral response.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
Metal oxide nanoparticles as well as preparation method and application thereof
ActiveCN110745784AImprove hydrogen production activitySimple preparation processOxygen/ozone/oxide/hydroxideNanotechnologyInorganic saltsMetal oxide nanoparticles
The invention discloses metal oxide nanoparticles as well as a preparation method and application thereof. Specifically, the invention discloses the metal oxide nanoparticles which ares prepared by the following steps: 1) dissolving an alkali inorganic salt into a reductive solvent, performing uniform mixing, adding an inorganic salt of a metal, and performing uniform stirring so as to obtain a homogeneous solution; 2) under a condition of an inert gas, performing a vacuum heating reaction, wherein the heating temperature is 150-600 DEG C; and 3) performing centrifugal separation on a solid, and performing washing and drying, so as to obtain metal oxide nanospheres. The method disclosed by the invention is simple, the prepared metal oxide nanospheres have a uniform particle size and a goodhydrogen generation ability, and the metal oxide is a metal oxide with the lowest valence.
Owner:SHENZHEN INST OF ADVANCED TECH
0D/2D composite photocatalysis material and preparation method and application thereof
InactiveCN110124697AFully crystallizedGood dispersionMaterial nanotechnologyCatalyst activation/preparationEthanol precipitationHigh pressure
The invention relates to the technical field of photocatalytic hydrogen generation, specifically to a 0D / 2D composite photocatalysis material and a preparation method and application thereof. The preparation method comprises the following steps: weighing a certain amount of ammonium molybdate and thioacetamide and dissolving the raw materials in deionized water to obtain a mixed solution, pouringthe mixed solution into a certain amount of a newly prepared Cu-In-Zn-S quantum dot solution, followed by uniform ultrasonic mixing; pouring the above solution into a high pressure reactor, placing the high pressure reactor into a drying oven of 180 DEG C, reacting for 16 h, and cooling to room temperature; and finally carrying out deionized water / ethanol ethanol precipitation / centrifugation circulatory washing on a compound for three times, drying the washed sample in a drying oven of 60 DEG C to obtain Cu-In-Zn-S / MoS2 nanocrystal. The experiment of visible light photocatalytic water splitting for hydrogen production proves that the prepared composite photocatalyst has a good photocatalytic activity.
Owner:JIANGSU UNIV
Supported photocatalyst and preparation method thereof
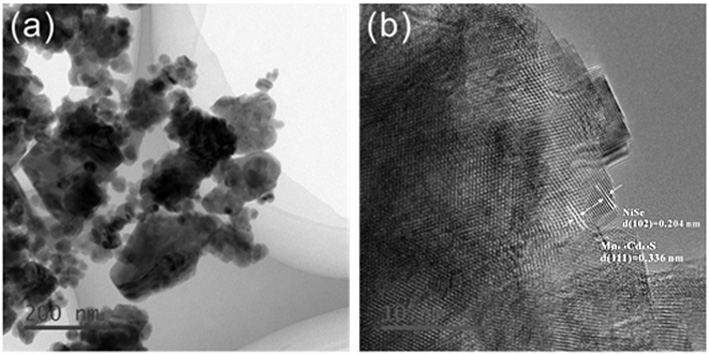
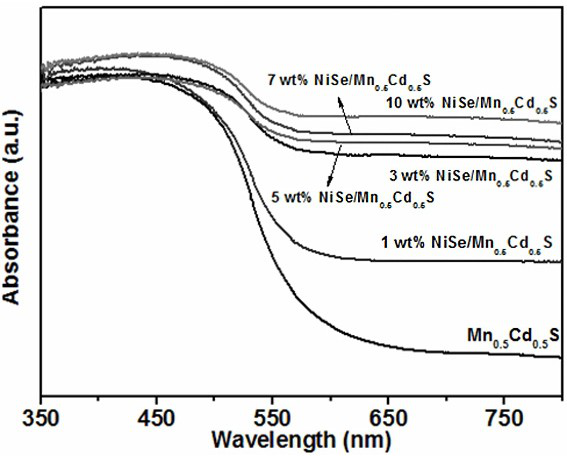
ActiveCN110302809AEasy transferEnhance photocatalytic hydrogen production activityPhysical/chemical process catalystsHydrogen productionHeterojunctionNickel sulfate hydrate
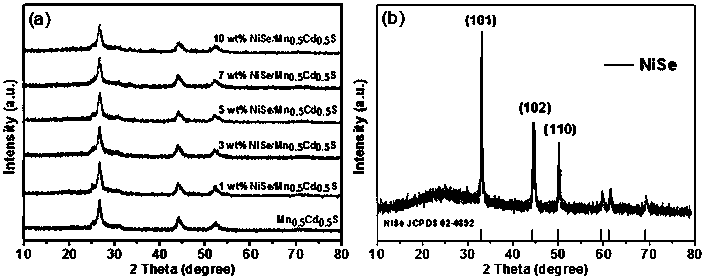
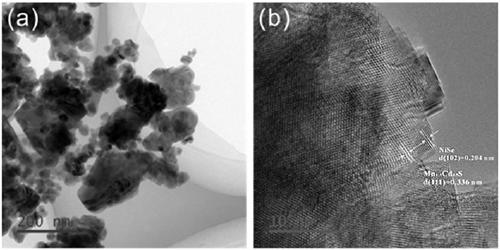
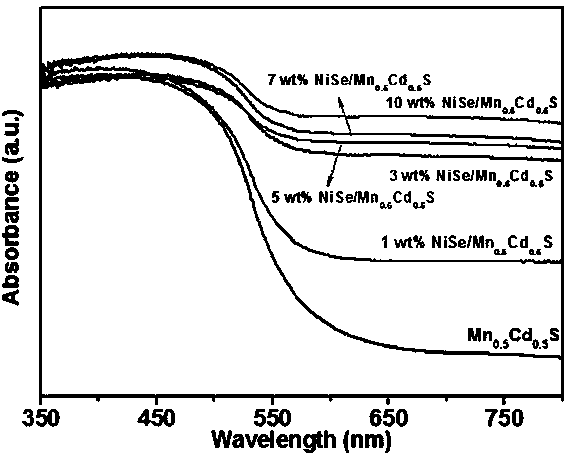
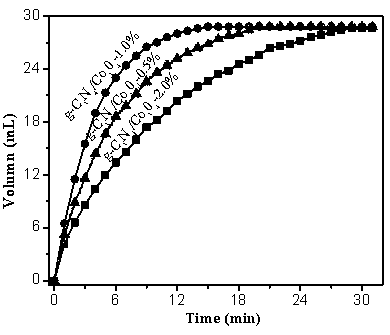
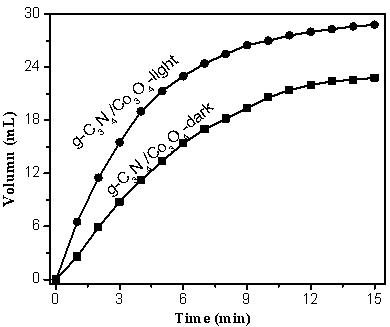
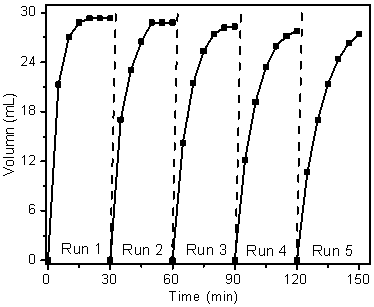
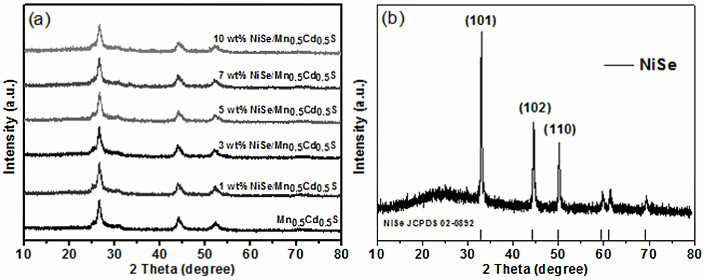
The invention discloses a supported photocatalyst NiSe / Mn0.5Cd0.5S and a preparation method thereof. The supported photocatalyst is prepared by firstly synthesizing a Mn0.5Cd0.5S solid solution by using a gentle one-step hydrothermal method, and by secondly, enabling the solid solution to react with nickel sulfate hexahydrate and sodium selenite. NiSe and Mn0.5Cd0.5S are tightly combined to form heterojunctions in the composite catalyst, migration of photon-generated carriers can be facilitated, combination of photon-generated electron holes is inhibited, and the photocatalysis hydrogen production activity of the Mn0.5Cd0.5S solid solution can be remarkably improved. The novel NiSe / Mn0.5Cd0.5S photocatalyst disclosed by the invention is simple to prepare, can be massively prepared, is highin photocatalysis activity and good in stability, and has wide application prospects in clean energy development.
Owner:FUZHOU UNIV
CdS/Ti-MCM-41 loaded platinum photo catalyst and its preparation method
InactiveCN100351013CHigh activityImprove stabilityMolecular sieve catalystsCatalyst activation/preparationPhotocatalytic reactionIon exchange
Owner:XI AN JIAOTONG UNIV
Ru monatomic and g-C3N4 composite photocatalyst and preparation method and application thereof
InactiveCN112973751ATake advantage ofEnhancing the efficiency of hydrogen production by splitting waterCatalyst activation/preparationHydrogen productionMaterial synthesisPhoto catalysis
The invention provides a Ru monatomic and g-C3N4 composite photocatalyst and a preparation method and application thereof, and belongs to the technical field of nano material synthesis. According to the device, Ru monatomic is loaded on the surface of g-C3N4 by utilizing a simple and rapid one-step calcination method, so that the Ru monatomic and g-C3N4 composite photocatalyst is formed; the photocatalyst can efficiently and stably produce hydrogen through water photolysis under visible light.
Owner:JIANGSU UNIV
Nb-Rh co-doped titanium dioxide nanorod photocatalyst, and preparation method and application thereof
ActiveCN108452802AEnhance photocatalytic hydrogen production activityEasy to prepareMaterial nanotechnologyHeterogenous catalyst chemical elementsChemistryTitanium dioxide
The invention discloses a Nb-Rh co-doped titanium dioxide nanorod photocatalyst, and a preparation method and application thereof. The photocatalyst of the invention has the following advantages: 1) the photocatalyst prepared in the invention has a one-dimensional rod structure and is effectively improved in photocatalytic hydrogen production activity compared with a common photocatalyst; 2) the preparation method of the photocatalyst is simple and can easily realize large-scale commercial production; 3) the photocatalyst can be reused, and the catalytic activity of the Nb-Rh co-doped titaniumdioxide nanorod photocatalyst is free of obvious decline after reuse 20 times or more; and 4) the Nb-Rh co-doped TiO2 nanorod photocatalyst has high catalytic activity, e.g., a Ti<0.996>Nb<0.002>Rh<0.002>O<2> nanorod photocatalyst has a hydrogen production rate of up to 7.58 mmol / g.h in photolysis of water, which is 194 times the hydrogen production rate (0.039 mmol / g.h) of TiO2 nanorods preparedunder the same conditions.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
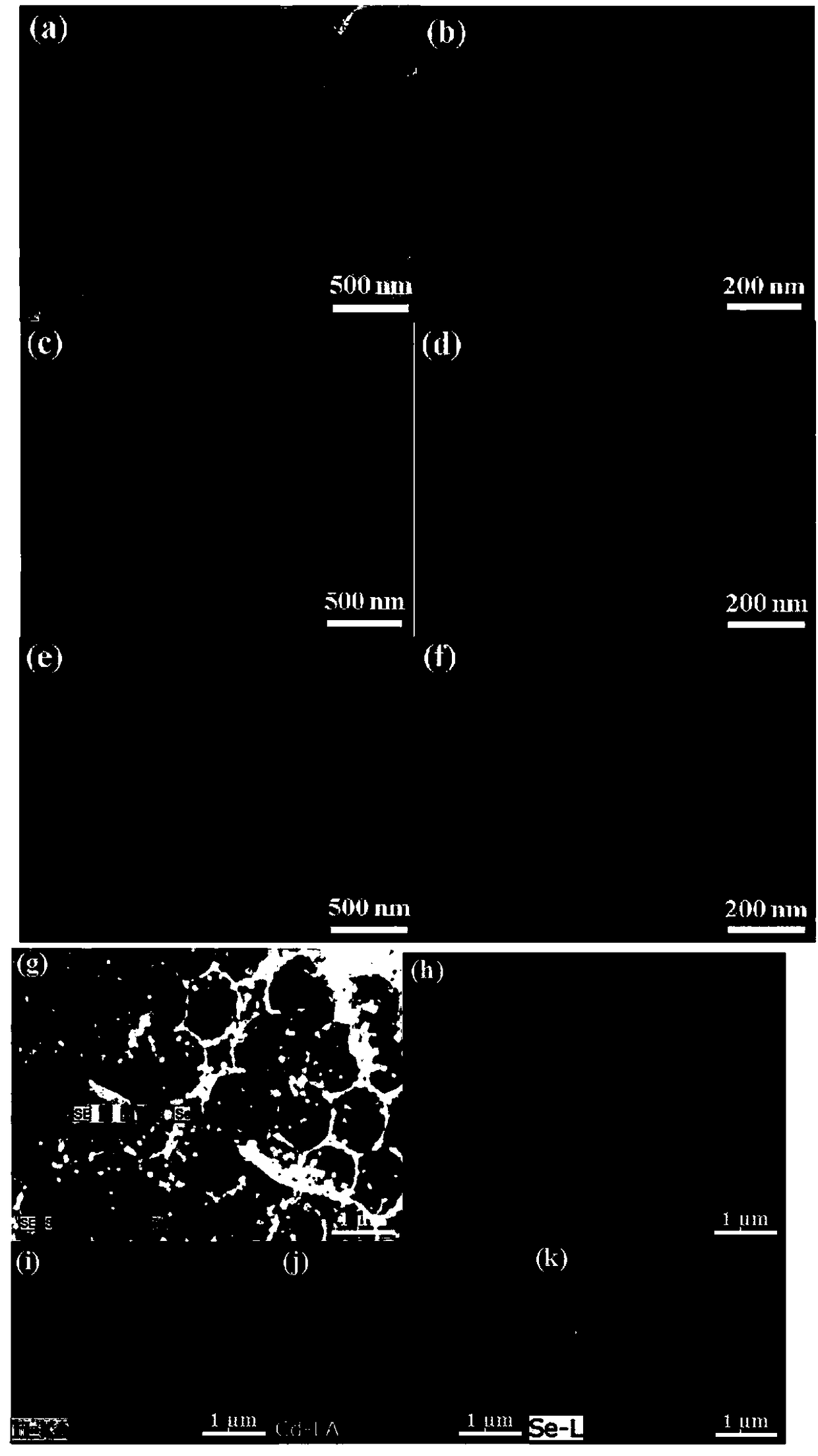
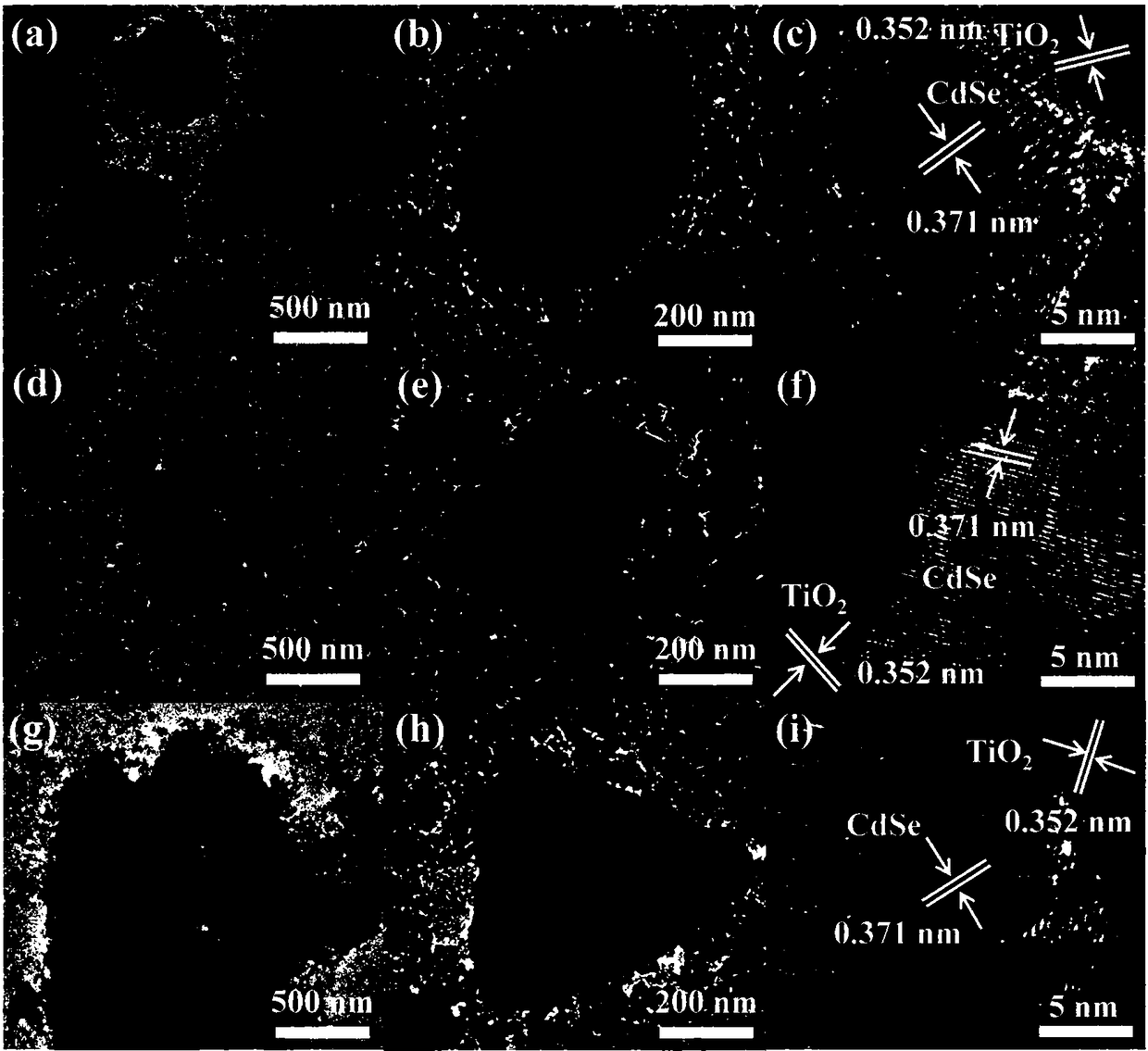
Preparation method and application for morphology-controlled CdSe-modified porous TiO2 material
InactiveCN108201890AImprove photoelectric performanceExcellent nonlinear optical propertiesHeterogenous catalyst chemical elementsHydrogen productionMaterial DesignMicrosphere
The invention discloses a preparation method and application for a morphology-controlled CdSe-modified porous TiO2 material. The preparation method comprises the following steps: Step I: synthesizingmonodisperse polystyrene microspheres (PS) with an average diameter of 500 nm; Step II: preparing CdO-TiO2 intermediate: preparing CdO-TiO2 intermediate by using the polystyrene microspheres obtainedin Step I) as a template in a sol-gel method; Step III: preparing nanosheets, nanorods and nano-conical CdSe particle-modified porous TiO2 photocatalyst in a two-step method. By means of the material,optically stimulated electrons can be transferred from a conduction band of CdSe to a conduction band of TiO2, the porous structure also improves active sites of the reaction, and the hydrogen production activity is improved. The growth-controlled composite material designed based on a reasonable material can obtain very good hydrogen evolution speed under visible light without any precious metalas a promoter.
Owner:ANYANG NORMAL UNIV
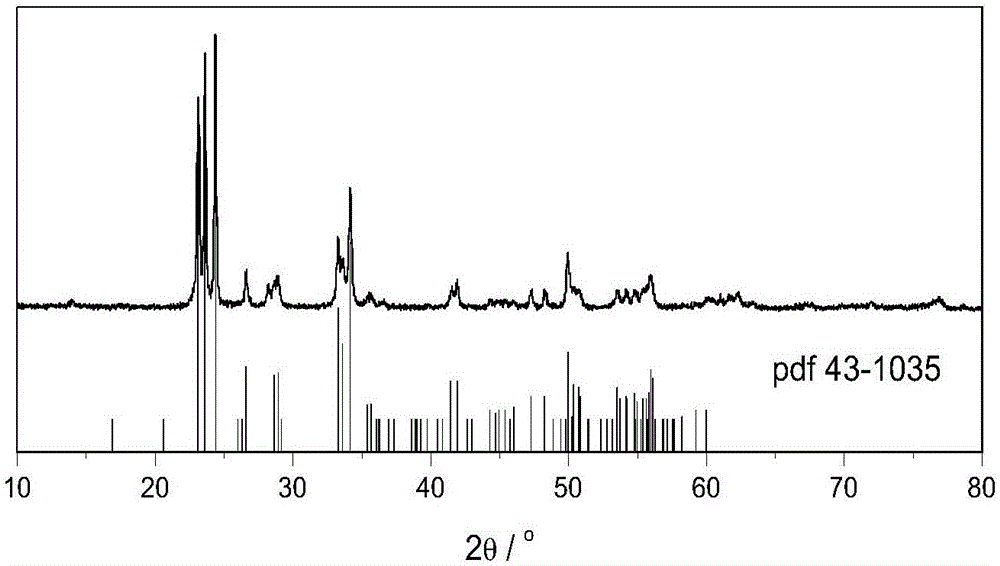
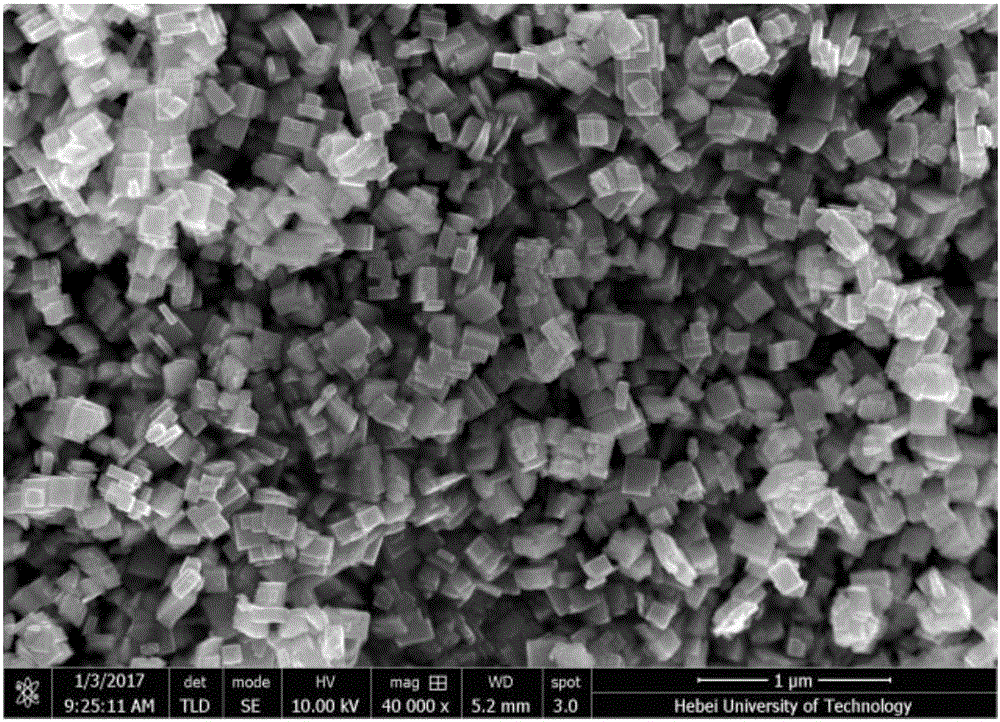
Preparation method of monoclinic system tungsten trioxide
InactiveCN106830087AGuaranteed decentralizationGuaranteed GranularityTungsten oxides/hydroxidesHeterogenous catalyst chemical elementsMicro nanoOrganic acid
The invention relates to a preparation method of monoclinic system tungsten trioxide. The preparation method of the monoclinic system tungsten trioxide comprises the following steps: (1) while stirring, adding nitric acid into sodium tungstate solution, stirring, then carrying out centrifugal separation, and collecting to obtain yellow precipitate; (2) transferring the yellow precipitate into a reactor, and adding deionized water, organic acid solution and polyethylene glycol solution; (3) then adding the nitric acid, and stirring for 0.5-2 hours; and (4) transferring the obtained slurry into an autoclave, sealing, then heating to the temperature of 160-250 DEG C, and continuously reacting for 8-20 hours, so that monoclinic system tungsten trioxide powder is obtained. The preparation method provided by the invention has the advantages that uniformly dispersed micro-nano tungsten trioxide crystal particles are obtained, and the obtained tungsten trioxide crystal particles have excellent photocatalytic performance.
Owner:HEBEI UNIV OF TECH
Method for preparing graphene-titanium dioxide composite photocatalyst
InactiveCN107899560AImprove hydrogen production activityInhibitory complexPhysical/chemical process catalystsHydrogen productionIce waterMicrowave
The invention discloses a method for preparing a graphene-titanium dioxide composite photocatalyst. The method comprises the following steps: 1, mixing graphite powder with NaNO3, carrying out a microwave reaction, and naturally cooling the obtained reaction product to room temperature; 2, adding a mixture obtained in step 1 into concentrated sulfuric acid in an ice water bath state, stirring andmixing the mixture and the concentrated sulfuric acid, and adding KMnO4; 3, placing a system obtained in step 2 in an oil bath, adding a hydrogen peroxide solution until the system generates no bubbles, washing the obtained precipitate, and carrying out vacuum drying on the precipitate to obtain graphite oxide; 4, adding the graphite oxide into an ethanol and water mixed solution, and carrying outultrasonic treatment; and 5, adding titanium dioxide, carrying out a stirring reaction at 150 DEG C, naturally cooling the obtained reaction product to room temperature, filtering the cooled product,washing the obtained precipitate, and carrying out vacuum drying to obtain the graphene-titanium dioxide composite photocatalyst. The graphene-titanium dioxide composite photocatalyst is prepared through a hydrothermal technology, and the hydrogen generation activity of the photocatalyst is high.
Owner:SHAANXI JUJIEHAN CHEM CO LTD
Ultraviolet-visible-near infrared responded PbS/TiO2 nanotube aggregation microsphere heterostructure as well as preparation method and application thereof
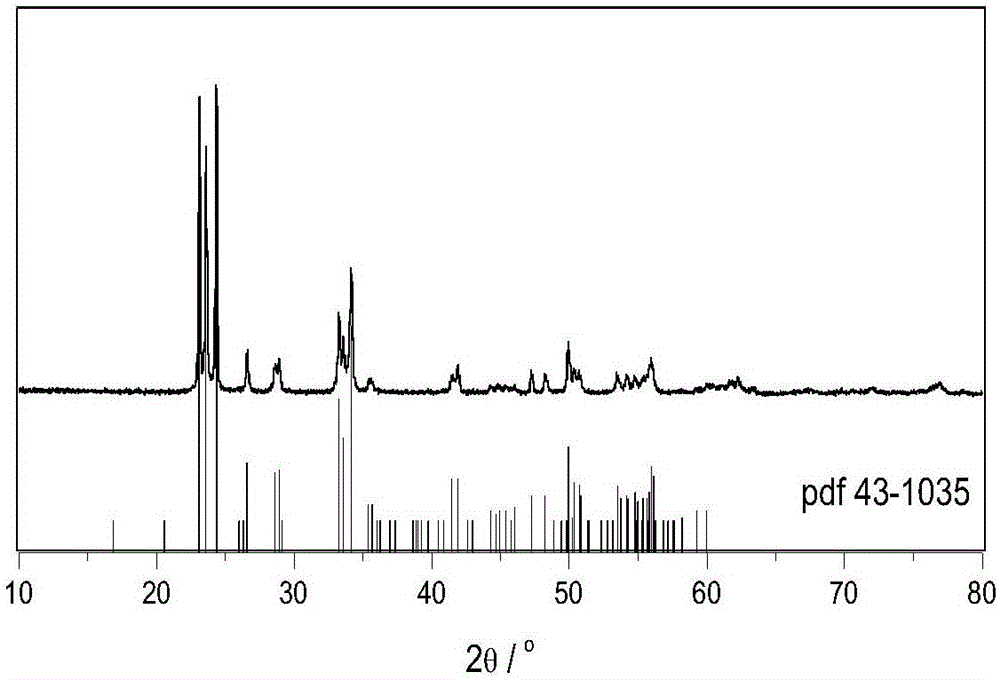
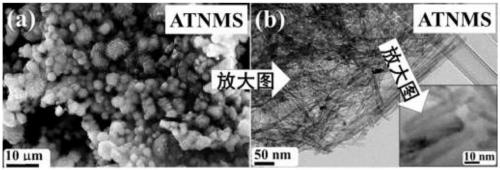
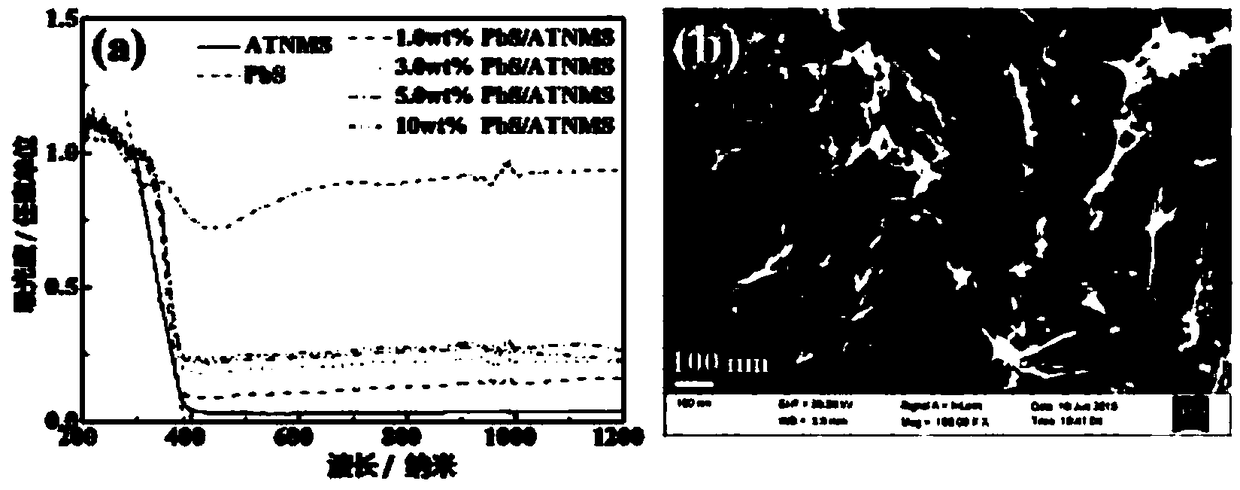
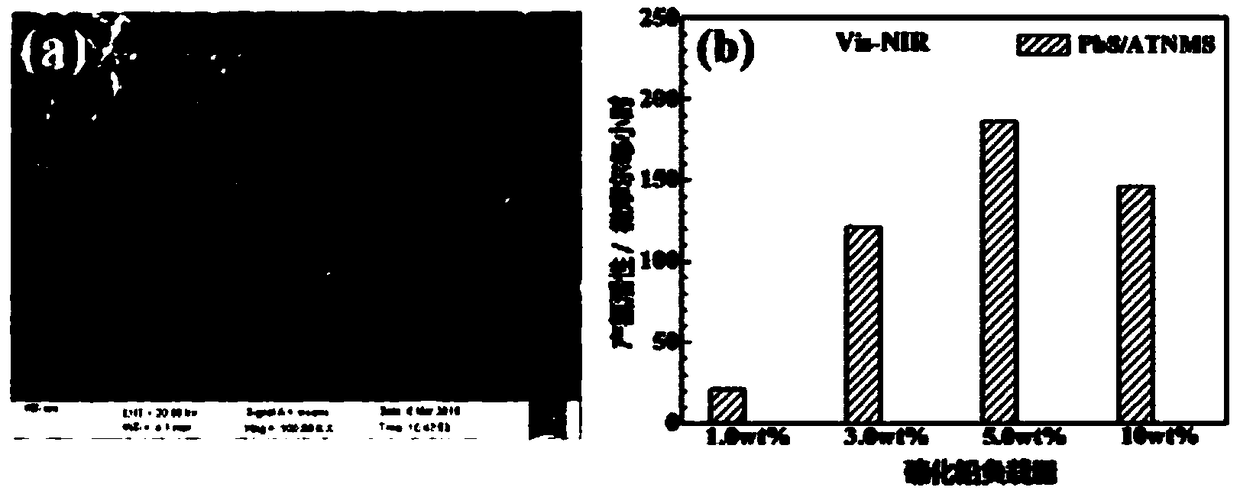
InactiveCN109364949AWide variety of sourcesLow costPhysical/chemical process catalystsHydrogen productionTio2 nanotubeUltraviolet
The invention discloses an ultraviolet-visible-near infrared responded PbS / TiO2 nanotube aggregation microsphere heterostructure as well as a preparation method and an application thereof. The ultraviolet-visible-near infrared responded PbS / TiO2 nanotube aggregation microsphere heterostructure provided by the invention has the characteristics that the heterostructure can be porous microsphere particles which are obtained by enabling PbS nanocrystals to be distributed on the surfaces of TiO2 nanotubes in TiO2 nanotube aggregation microspheres, wherein the particle diameter range of each PbS nanocrystal is 25nm to 36nm, the particle size of the TiO2 nanotube is not more than 10nm, and the diameter distribution of the aggregation microspheres is within the range of 0.2 micron to 7 micron. Thebonding force between the PbS and the TiO2 nanotube in the heterostructure is strong, and the heterostructure has good ultraviolet-visible-near infrared responded photocatalytic hydrogen production activity, and higher hydrogen production is almost realized in the full spectrum range.
Owner:SUZHOU INSTITUE OF WUHAN UNIV +1
Efficient nanocrystal colloidal hydrogen production catalyst and preparation method thereof
InactiveCN109701581AImprove hydrogen production activityAvoid reunionPhysical/chemical process catalystsHydrogen productionHydrothermal synthesisUltrasonic dispersion
The invention discloses an efficient nanocrystal colloidal hydrogen production catalyst and a preparation method thereof. The technical scheme is that a cobalt dichloride solution, a g-C3N4 powder ultrasonic dispersion solution and a PVP colloidal solution are mixed according to a certain ratio under the condition of a certain temperature, and then, a nanocrystal colloidal material with g-C3N4 / Co3O4 photoresponse reinforced catalytic hydrogen production performance is prepared by using a hydrothermal synthesis method. The preparation method provided by the invention is simple in operation, lowin cost, safe and environment-friendly; and the prepared g-C3N4 / Co3O4 nanocrystal colloidal material has relatively small nanocrystal size, good dispersibility and efficient photoresponse reinforcedcatalytic hydrogen production performance.
Owner:XIAN TECHNOLOGICAL UNIV
A kind of supported photocatalyst and preparation method thereof
ActiveCN110302809BEasy transferEnhance photocatalytic hydrogen production activityPhysical/chemical process catalystsHydrogen productionHeterojunctionHydration reaction
The invention discloses a loaded photocatalyst NiSe / Mn 0.5 Cd 0.5 S and preparation method thereof, it is to adopt mild one-step hydrothermal method to synthesize Mn earlier 0.5 Cd 0.5 S solid solution, and then react it with nickel sulfate hexahydrate and sodium selenite. NiSe and Mn in the composite catalyst 0.5 Cd 0.5 S is closely combined to form a heterojunction, which is beneficial to the migration of photogenerated carriers and inhibits the recombination of photogenerated electrons and holes, making Mn 0.5 Cd 0.5 The photocatalytic hydrogen production activity of S solid solution was significantly improved. The novel NiSe / Mn that the present invention uses 0.5 Cd 0.5 S photocatalyst has the advantages of simple preparation, mass preparation, high photocatalytic activity and good stability, which makes it have broad application prospects in the development of clean energy.
Owner:FUZHOU UNIV
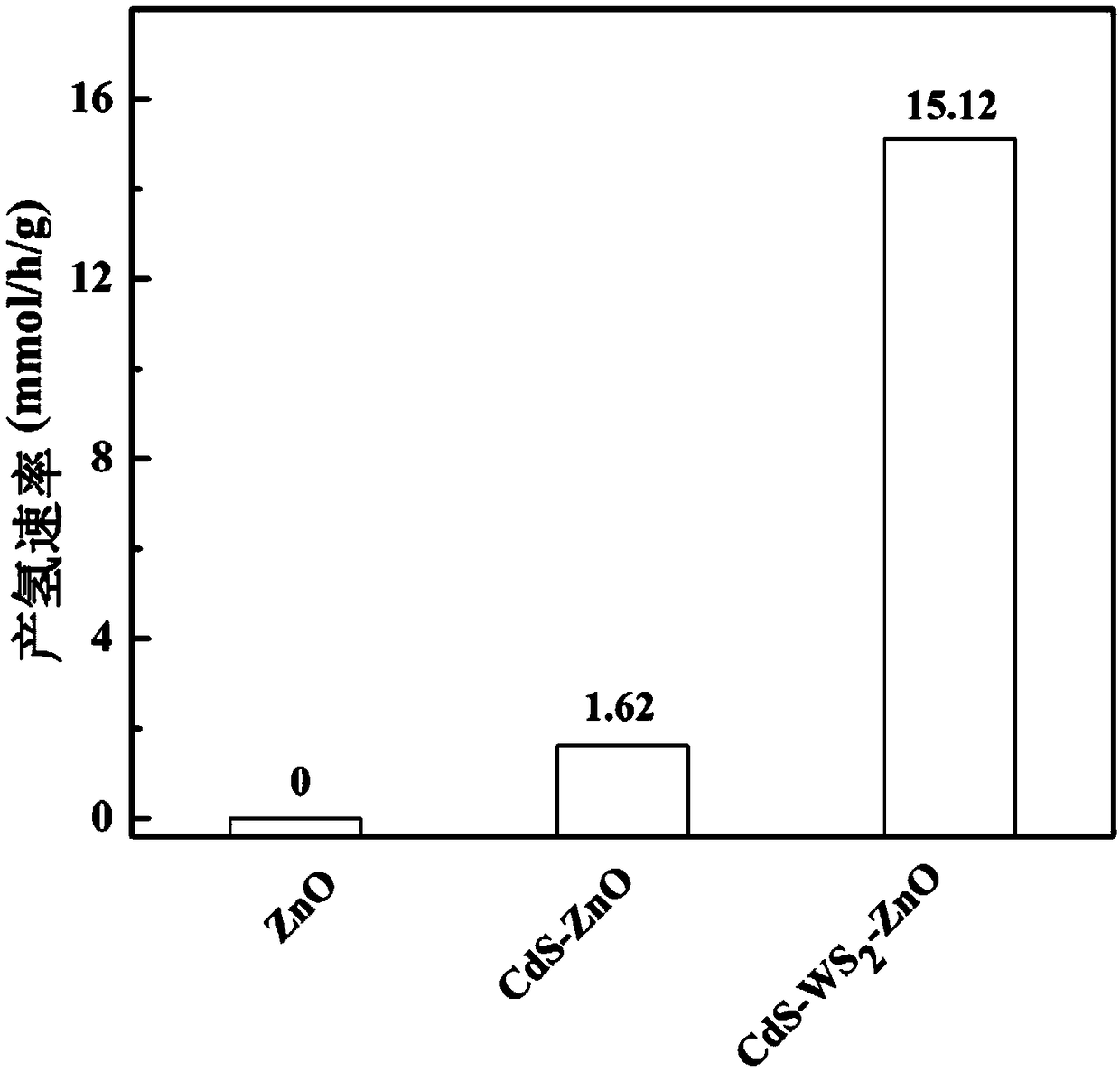
High-efficiency hydrogen-production ZnO core-shell nanorod array photocatalyst, preparation method and application thereof
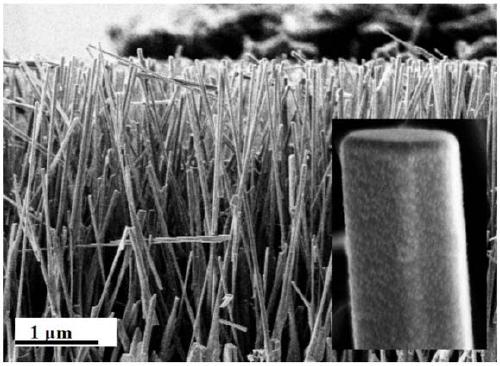
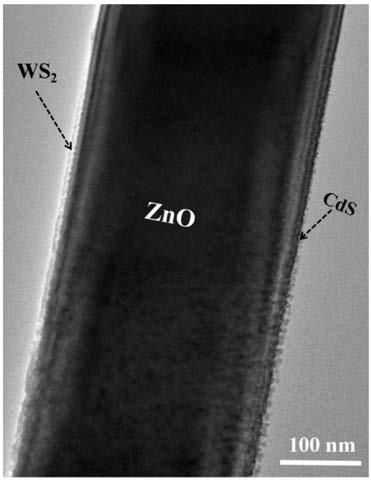
ActiveCN109289875AImprove hydrogen production activityCatalyst activation/preparationHydrogen productionNanoparticleCharge carrier
The invention discloses a high-efficiency hydrogen-production ZnO core-shell nanorod array photocatalyst. The high-efficiency hydrogen-production ZnO core-shell nanorod array photocatalyst comprises an insulation substrate, the ZnO nanorod arrays are distributed on the substrate, the ZnO nanorod arrays are composed of several ZnO nano rods; the outer layers of the ZnO nanorods are a WS2 films, which form a ZnO-WS2 composite system; the outer layers of the ZnO-WS2 composite system is loaded with CdS nanoparticles to form the ZnO-WS2-CdS core-shell nanorod arrays. The invention also provides a preparation method of the photocatalyst and an application of the photocatalyst for hydrogen production under visible light catalysis. The photocatalyst of the present invention has an effective electron transfer energy level, achieves rapid carrier separation, and increases hydrogen production activity.
Owner:SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com