Nb-Rh co-doped titanium dioxide nanorod photocatalyst, and preparation method and application thereof
A photocatalyst and titanium dioxide technology, which is applied in the field of photocatalytic materials, achieves the effects of simple preparation method, easy large-scale commercial production, and improved photocatalytic hydrogen production activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0069] Niobium pentachloride and rhodium trichloride hydrate with a rhodium content of 40% were dissolved in ethanol respectively to prepare 0.05M niobium pentachloride ethanol solution and 0.01M rhodium trichloride ethanol solution.
Embodiment 1
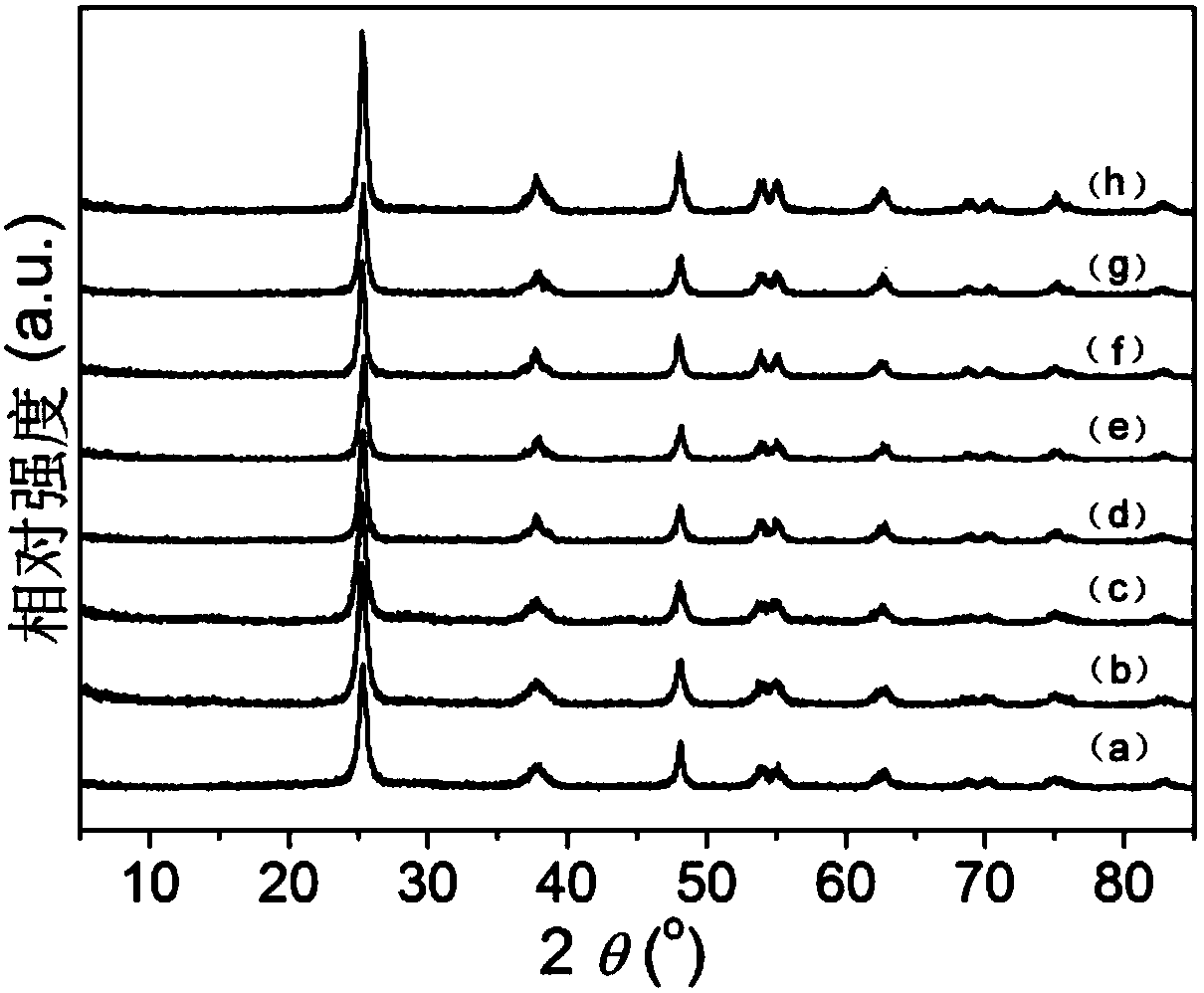
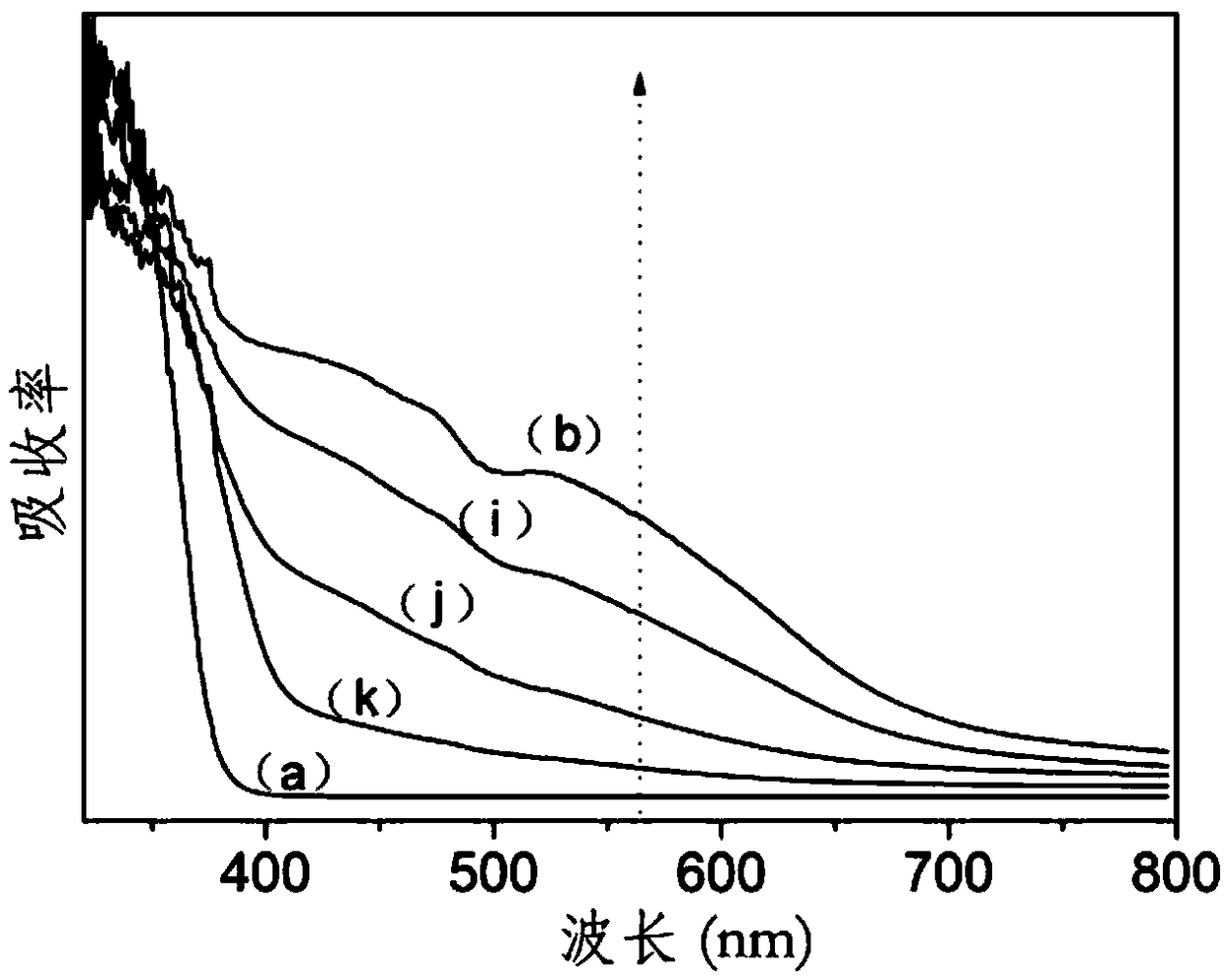
[0071] About 1mL of hydrochloric acid, 0.13mL of the 0.05M niobium pentachloride prepared in the above preparation example 1, 1mL of the 0.01M rhodium trichloride prepared in the above preparation example 1 and 3.4g of butyl titanate were added to 20mL of anhydrous In the ethanol solution, stir to accelerate the dissolution and form a transparent solution, then add 3-5mL of high-purity water, and continue stirring until a gel is formed. The gel was poured into 10M NaOH solution and stirred to disperse, filtered and redispersed into 10M NaOH solution, and then hydrothermally reacted at 150°C for 100h. The cooled product was washed several times with pure water, dilute nitric acid, pure water, and absolute ethanol in sequence, then dried in an oven at 70°C for 20 hours, and then put into a muffle furnace for calcination at 450°C for 4 hours to obtain Nb-Rh co-doped Doped TiO 2 Nanorods - Ti 0.999 Nb 0.0005 Rh 0.0005 o 2 Nano stave.
[0072] The results show that: the Nb-Rh...
Embodiment 2
[0075] About 1mL of hydrochloric acid, 0.13mL of the 0.05M niobium pentachloride prepared in the above preparation example 1, 10mL of the 0.01M rhodium trichloride prepared in the above preparation example 1 and 3.4g of butyl titanate were added to 20mL of anhydrous In the ethanol solution, stir to accelerate the dissolution and form a transparent solution, then add 3-5mL of high-purity water, and continue stirring until a gel is formed. The gel was poured into 10M NaOH solution and stirred to disperse, filtered and redispersed into 10M NaOH solution, and then hydrothermally reacted at 150°C for 100h. The cooled product was washed several times with pure water, dilute nitric acid, pure water, and absolute ethanol in sequence, then dried in an oven at 70°C for 20 hours, and then put into a muffle furnace for calcination at 450°C for 4 hours to obtain Nb-Rh co-doped Doped TiO 2 Nanorods - Ti 0.99 Nb 0.0005 Rh 0.0095 o 2 Nano stave.
[0076] The results show that: the Nb-Rh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com