Fluorine-substituted Zn/Co porphyrin-based conjugated organic polymer as well as preparation method thereof and application thereof
A conjugated polymer and porphyrin-based technology is applied in the field of organic polymer-based photocatalytic materials and hydrogen production applications, which can solve the problem of affecting the photocatalytic effect of sunlight utilization efficiency and underutilizing porphyrin-based conjugated supramolecules. There are no patent publications or research reports on light absorption performance, visible/near-infrared light-driven hydrogen production, etc., to achieve the effects of good light absorption performance, low energy consumption cost, and reduced charge recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
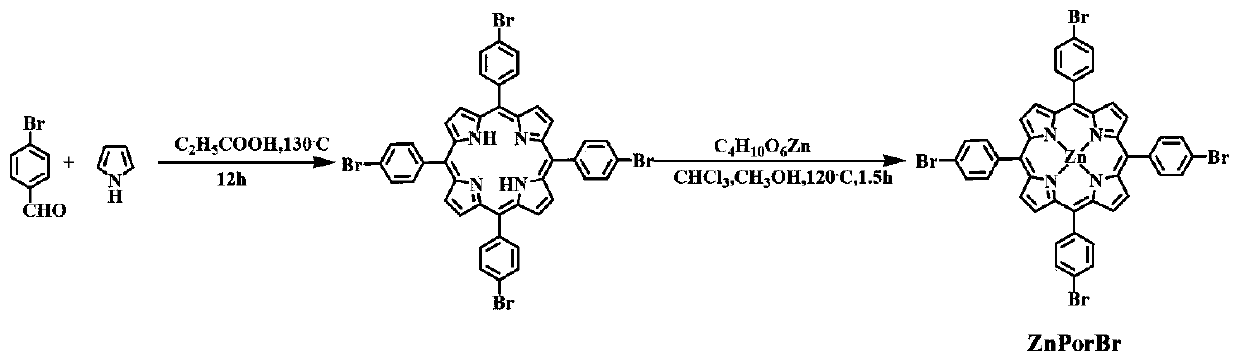
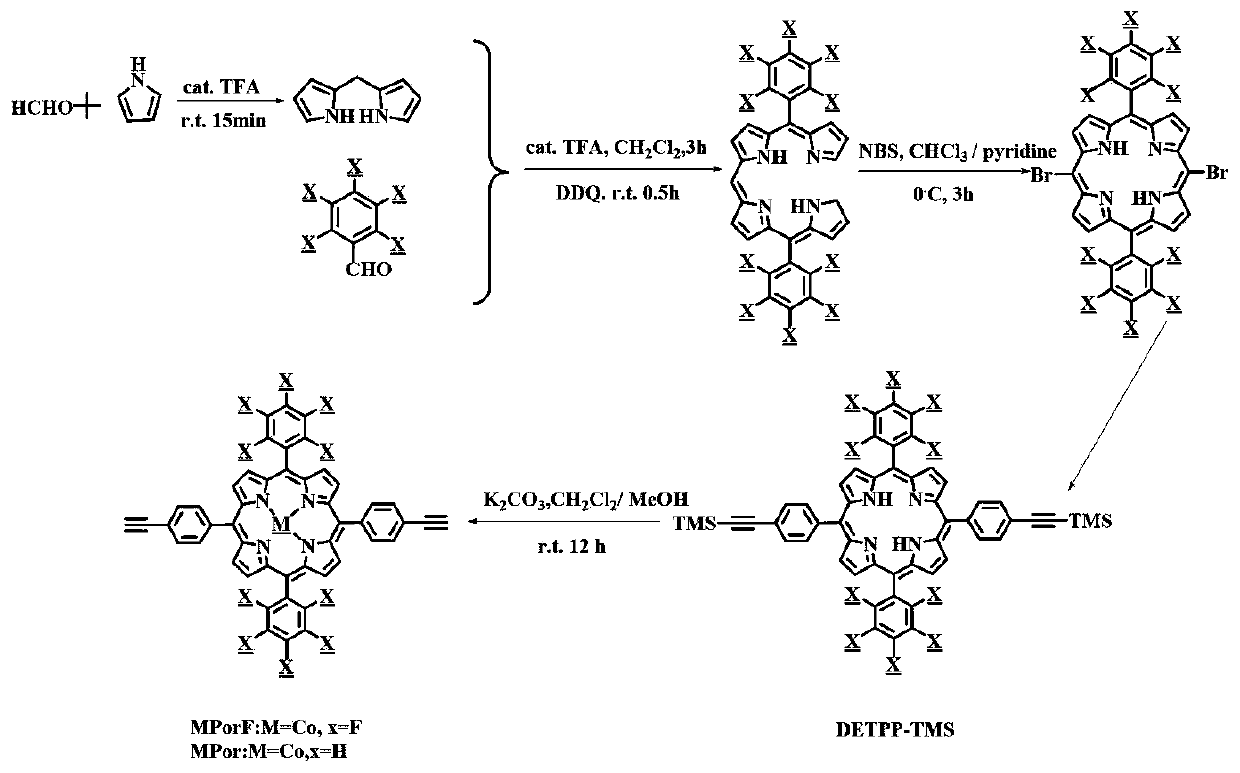
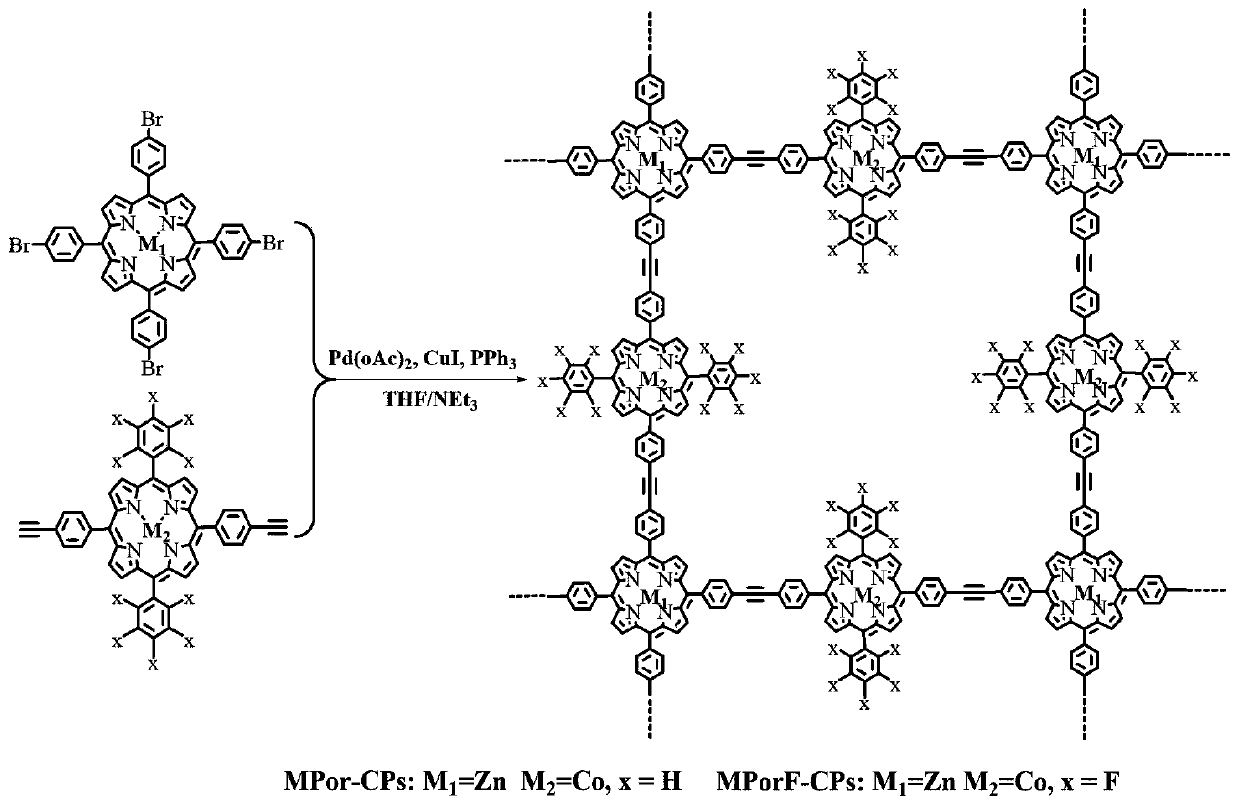
[0037] first use Figure 1-3 Synthetic route to synthesize MPorF-CPs polymer.
[0038] Wherein, when x=H, the product is marked as MPor-CPs, which is used as a comparative reference in this study. Figure 4 Show MPorF-CPs polymer has better light absorption properties than MPor-CPs. Get MPorF-CPs polymer 10mg and join in the TEOA aqueous solution of 50mL (TEOA volume concentration 15%), before testing, this dispersion system ultrasonic treatment 5min, and remove the air in the reactor, after light for a period of time, adopt gas chromatography ( GC, SP6890, TCD detector, 5A molecular sieve, argon as carrier gas) to detect the hydrogen production of the system. Depend on Figure 5 It can be seen that the hydrogen production activity after visible-near-infrared (Vis-NIR) irradiation for 1 h is 69 μmol h -1 . Figure 6 showed that the MPorF-CPs polymer achieved 10.4%, 7.8%, 8.2%, 8.7%, 4.8%, 2.9% and 0.76% of the apparent quantum The yield is higher than the apparent quantu...
Embodiment 2
[0040] first use Figure 1-3 Synthetic route to synthesize MPorF-CPs polymer.
[0041] Wherein, when x=H, the product is marked as MPor-CPs, which is used as a comparative reference in this study. Figure 4 Show MPorF-CPs polymer has better light absorption properties than MPor-CPs. Get MPorF-CPs polymer 20mg and join in the TEOA aqueous solution of 50mL (TEOA volume concentration 15%), before testing, this dispersion system ultrasonic treatment 5min, and remove the air in the reactor, after light for a period of time, adopt gas chromatography ( GC, SP6890, TCD detector, 5A molecular sieve, argon as carrier gas) to detect the hydrogen production of the system. Depend on Figure 5 It can be seen that the hydrogen production activity after visible-near-infrared (Vis-NIR) irradiation for 1 h is 138 μmol h -1 , indicating that the visible light photocatalytic hydrogen production performance tends to increase with the increase of the amount of MPorF-CPs polymer. Figure 6 show...
Embodiment 3
[0043] first use Figure 1-3 Synthetic route to synthesize MPorF-CPs polymer.
[0044] Wherein, when x=H, the product is marked as MPor-CPs, which is used as a comparative reference in this study. Figure 4 Show MPorF-CPs polymer has better light absorption properties than MPor-CPs. Get MPorF-CPs polymer 30mg and join in the TEOA aqueous solution of 50mL (TEOA volume concentration 15%), before testing, this dispersion system ultrasonic treatment 5min, and remove the air in the reactor, after light for a period of time, adopt gas chromatography ( GC, SP6890, TCD detector, 5A molecular sieve, argon as carrier gas) to detect the hydrogen production of the system. Depend on Figure 5 It can be seen that the hydrogen production activity after visible-near-infrared (Vis-NIR) irradiation for 1 h is 165 μmol h -1 , indicating that the visible-light photocatalytic hydrogen production performance of MPor-CPs polymers further increases with the increase of polymer dosage. Figure 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com