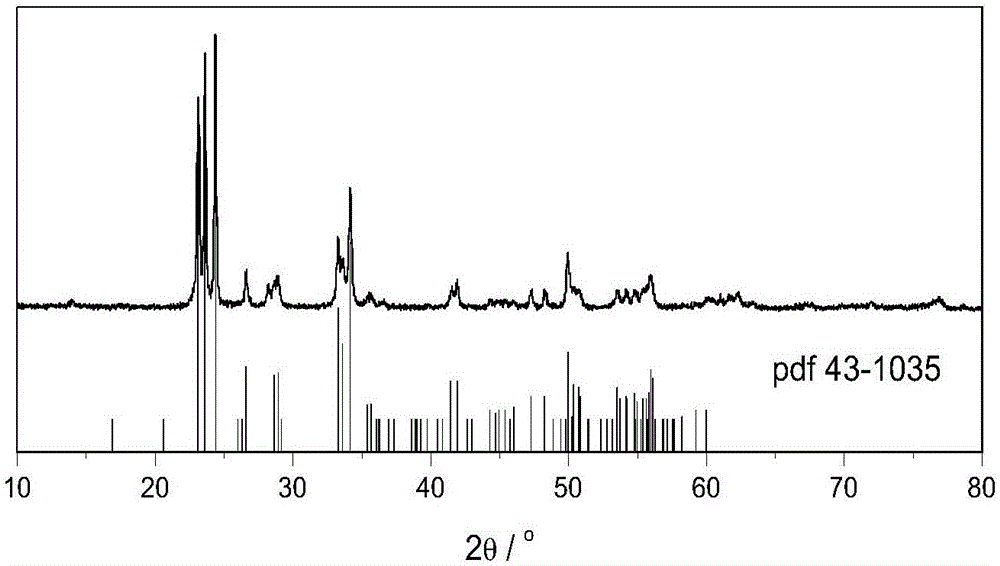
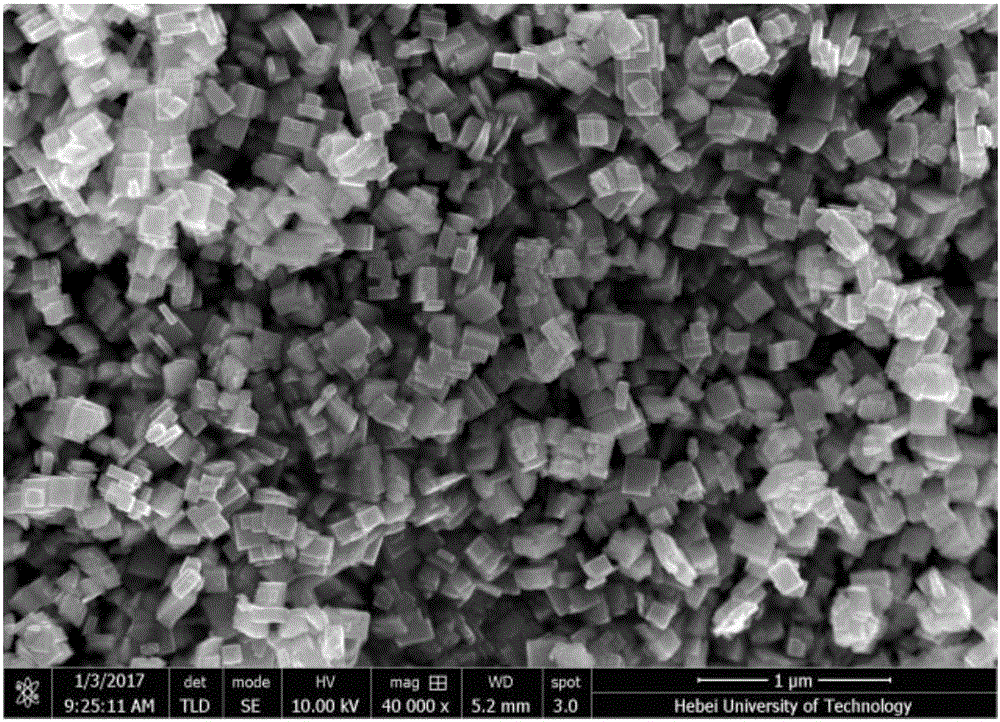
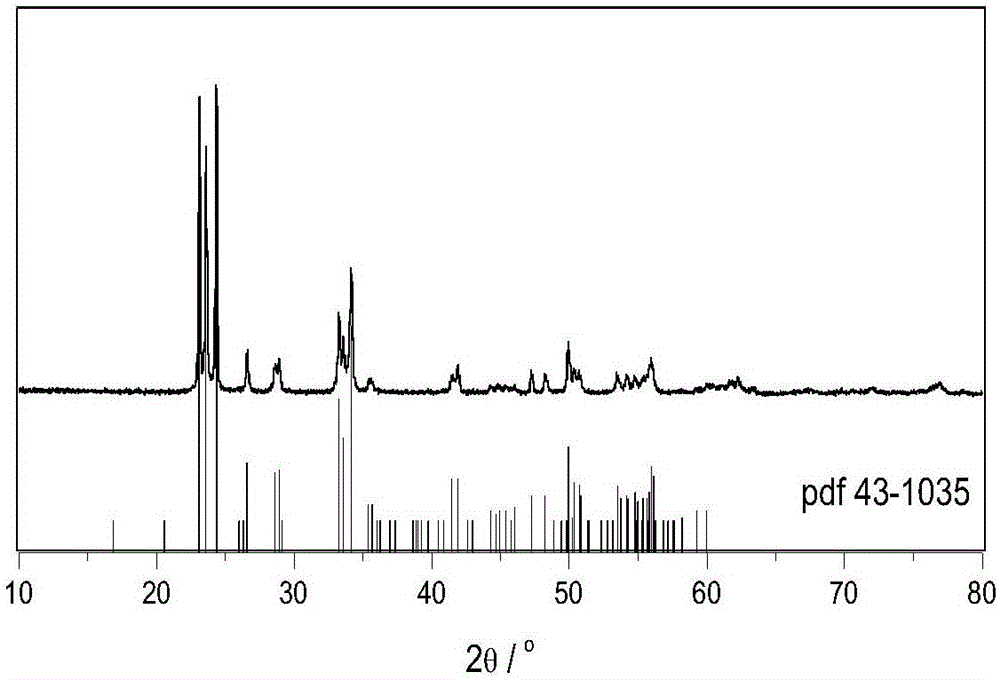
Preparation method of monoclinic system tungsten trioxide
A technology of tungsten trioxide and sodium tungstate, applied in chemical instruments and methods, tungsten oxide/tungsten hydroxide, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of poor dispersion and change of crystal shape Improve the hydrogen production activity, ensure the integrity, and achieve the effects of excellent photocatalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Weigh 5gNa 2 WO 4 .2H 2 O, add deionized water to prepare an aqueous solution with a mass concentration of 10%, according to NHO 3 Add nitric acid solution with a mass concentration of 63% at a molar ratio of 2:1 to tungsten atoms to obtain a light yellow precipitate, stir for 0.5 hours, and the precipitate turns into bright yellow. After centrifugation, a yellow precipitate was collected.
[0030] (2) Transfer the yellow precipitate obtained in (1) into a beaker, add deionized water with a mass ratio of 6:1 to the precipitate, and stir to form a slurry. Simultaneously weigh citric acid at a ratio of 2:1 to tungsten atom molar ratio, add water to prepare an aqueous solution with a mass concentration of 20%; weigh polyethylene glycol 2000 at a ratio of 0.5:1 to tungsten atom mass ratio, add water It is prepared into an aqueous solution with a mass concentration of 20%, and the two are sequentially added to the aforementioned slurry.
[0031] (3) Press NHO 3 The...
Embodiment 2
[0035] (1) Weigh 5gNa 2 WO 4 .2H 2 O, add deionized water to prepare an aqueous solution with a mass concentration of 10%, press HNO 3 Add nitric acid solution with a mass concentration of 63% at a molar ratio of 2:1 to tungsten atoms to obtain a light yellow precipitate, stir for 0.5 hours, and the precipitate turns into bright yellow. After centrifugation, a yellow precipitate was collected.
[0036] (2) Transfer the yellow precipitate obtained in (1) into a beaker, add deionized water with a mass ratio of 6:1 to the precipitate, and stir to form a slurry. At the same time, weigh L-tartaric acid in a ratio of 1:1 to the tungsten atom molar ratio, add water to prepare an aqueous solution with a mass concentration of 20%; weigh polyethylene glycol 2000 in a ratio of 0.3:1 to the tungsten atom mass ratio, add water It is prepared into an aqueous solution with a mass concentration of 20%, and the two are sequentially added to the aforementioned slurry.
[0037] (3) Press HN...
Embodiment 3
[0041] Take by weighing 4g copper nitrate, be mixed with deionized water and be that mass concentration is the aqueous solution of 10%, be 1.1:1 to take by weighing oxalic acid by the mol ratio of oxalic acid and copper, be mixed with deionized water that mass concentration is the oxalic acid aqueous solution of 15%, The latter was added to the former to form a copper oxalate precipitate, which was deposited for 20 minutes and then filtered. Weigh chromium nitrate according to the molar ratio of chromium to copper as 1:1, prepare a chromium nitrate aqueous solution with a mass concentration of 10% with deionized water, add copper oxalate precipitate to it, stir mechanically to form a slurry, and stir At the same time, heat it on a water bath at 85°C to dehydrate the slurry, and then move it into an oven to dry after it becomes thick. Grind the dried powder with a mortar, roast in a nitrogen atmosphere at 600°C for 5h, at 800°C for 3h, at 950°C for 3h, and at 1000°C for 3h to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com