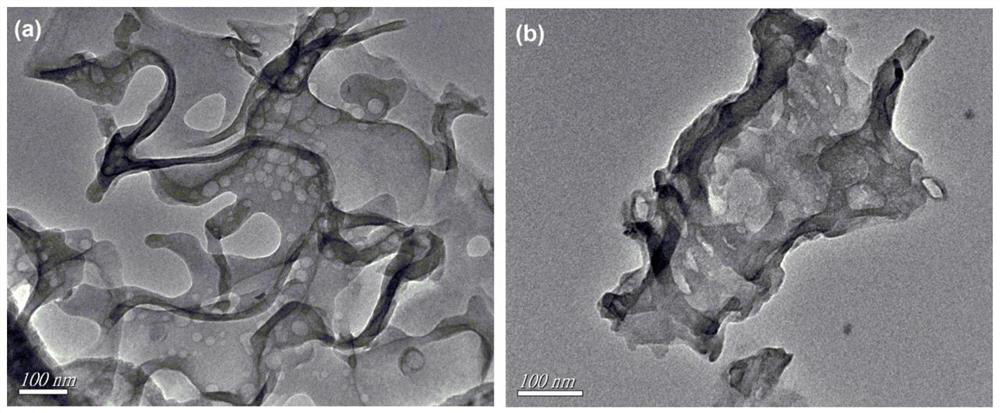
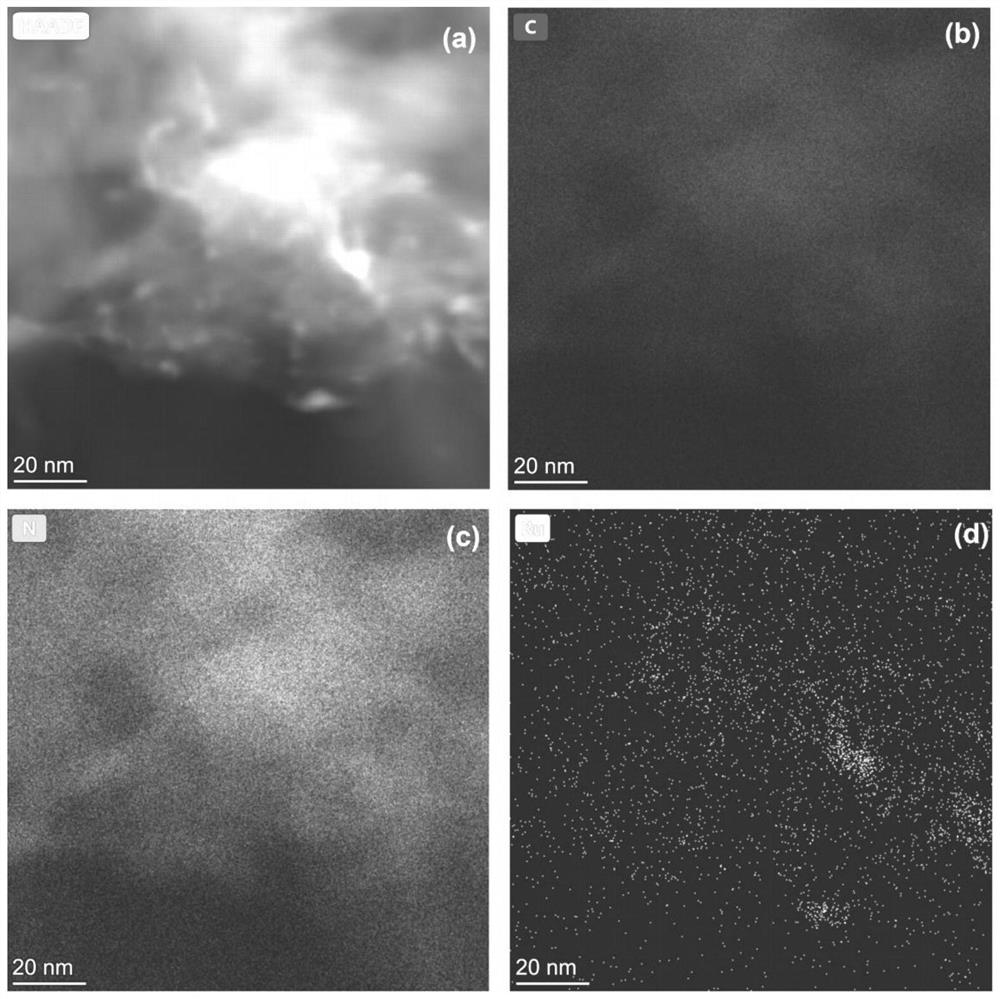
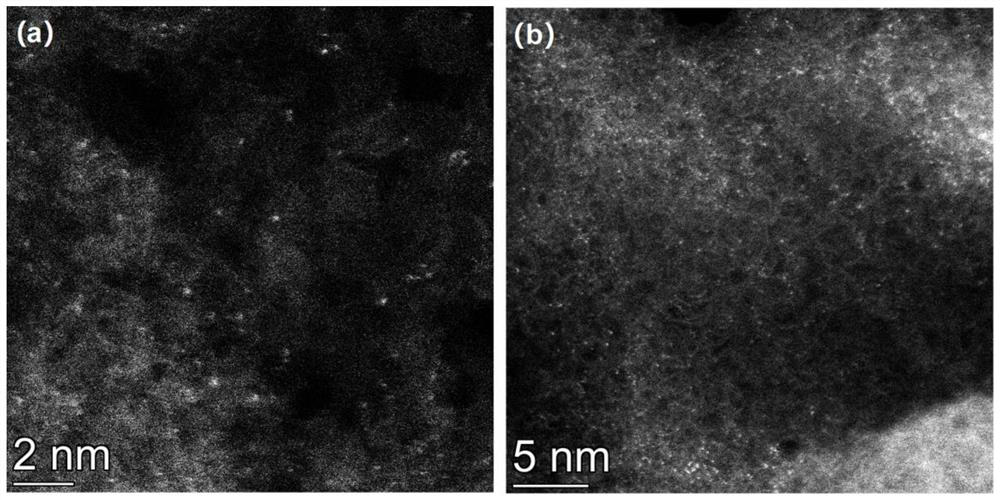
Ru monatomic and g-C3N4 composite photocatalyst and preparation method and application thereof
A g-c3n4, catalyst technology, applied in catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of reduced photocatalytic activity, affecting the use of photocatalysts, and inability to maintain stable performance. Enhanced absorption capacity, good optical properties and surface adsorption properties, and the effect of enriching surface active sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of RuCN-0.05% photocatalyst and its photolysis of water to produce hydrogen
[0033] Weigh 20g of urea, dissolve it in deionized water to form solution A; weigh a certain amount of ruthenium acetylacetonate, metal Ru and g-C 3 N 4The mass ratio is 0.05:100, dissolved in ethanol to form solution B; pour B into A, stir magnetically for 10 minutes, stir and dry the mixed solution in an oil bath at 90°C, and after it is completely dry, put the dried sample into a glass Grind it into powder in a mortar, pour it into a 50ml crucible, wrap tin foil on the outside of the crucible, and heat it in a muffle furnace from room temperature to 500°C at a rate of 5°C / min for 3 hours. After cooling down to room temperature naturally, the RuCN-0.05% photocatalyst was obtained.
[0034] The photocatalytic reaction was carried out in a closed glass reaction system, and 50mg RuCN-0.05% composite catalyst was uniformly dispersed in 100ml, 10vol% triethanolamine (TEOA...
Embodiment 2
[0035] Example 2: Preparation of RuCN-0.1% photocatalyst and its photolysis of water to produce hydrogen
[0036] Weigh 20g of urea, dissolve it in deionized water to form solution A; weigh a certain amount of ruthenium acetylacetonate, metal Ru and g-C 3 N 4 The mass ratio is 0.1:100, dissolved in ethanol to form solution B; pour B into A, stir magnetically for 10 minutes, stir and dry the mixed solution in an oil bath at 90°C, and after it is completely dry, put the dried sample into a glass Grind it into powder in a mortar, pour it into a 50ml crucible, wrap tin foil on the outside of the crucible, and heat it in a muffle furnace from room temperature to 525°C at a rate of 5°C / min for 4 hours. After naturally cooling down to room temperature, the RuCN-0.1% photocatalyst was obtained.
[0037] The photocatalytic reaction was carried out in a closed glass reaction system, and 50mg RuCN-0.1% composite catalyst was uniformly dispersed in 100ml, 10vol% triethanolamine (TEOA) a...
Embodiment 3
[0038] Example 3: Preparation of RuCN-0.5% photocatalyst and its photolysis of water to produce hydrogen
[0039] Weigh 20g of urea, dissolve it in deionized water to form solution A; weigh a certain amount of ruthenium acetylacetonate, metal Ru and g-C 3 N 4 The mass ratio is 0.5:100, dissolved in ethanol to form solution B; pour B into A, stir magnetically for 10 minutes, stir and dry the mixed solution in an oil bath at 90°C, and after it is completely dry, put the dried sample into a glass Grind it into powder in a mortar, pour it into a 50ml crucible, wrap tin foil on the outside of the crucible, and heat it in a muffle furnace from room temperature to 500°C at a rate of 5°C / min for 3 hours. After cooling down to room temperature naturally, the RuCN-0.5% photocatalyst was obtained.
[0040] The photocatalytic reaction was carried out in a closed glass reaction system, and 50mg RuCN-0.5% composite catalyst was uniformly dispersed in 100ml, 10vol% triethanolamine (TEOA) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com