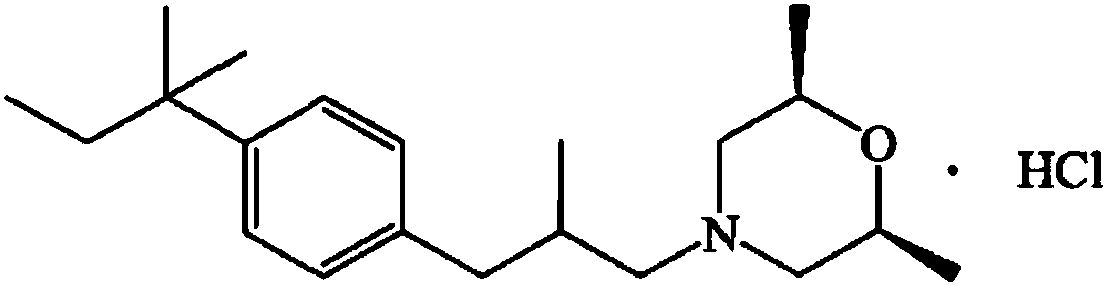
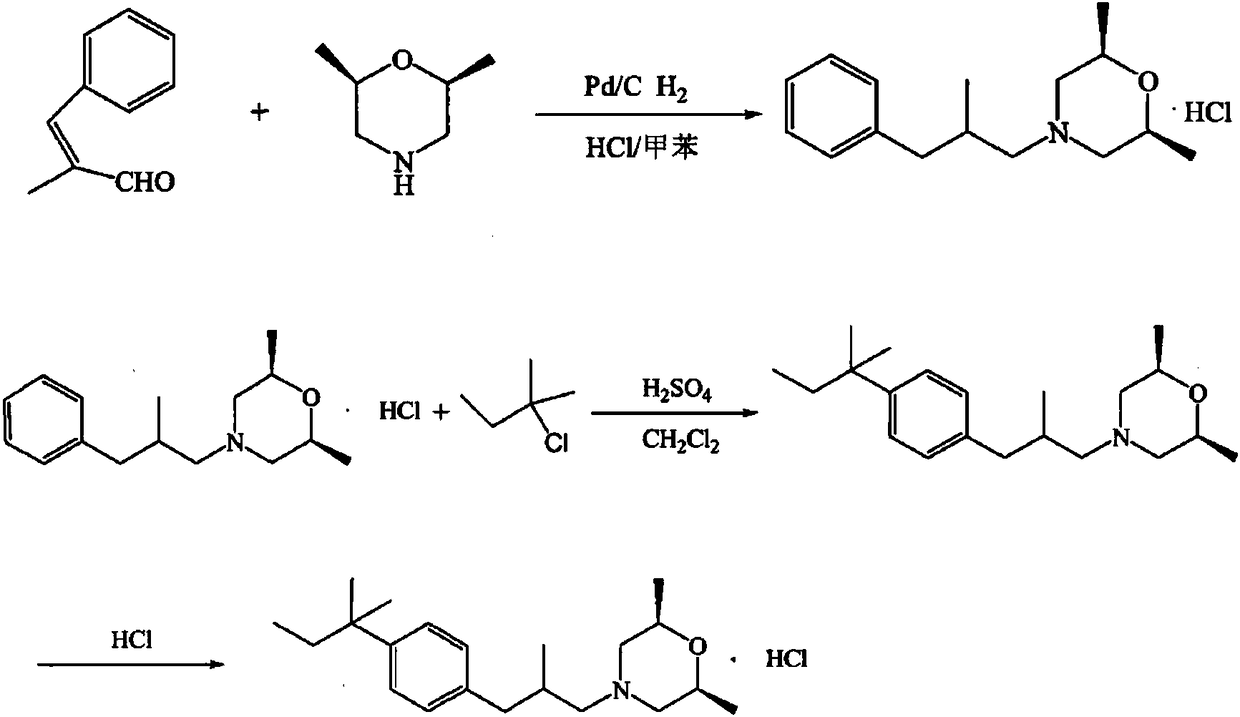
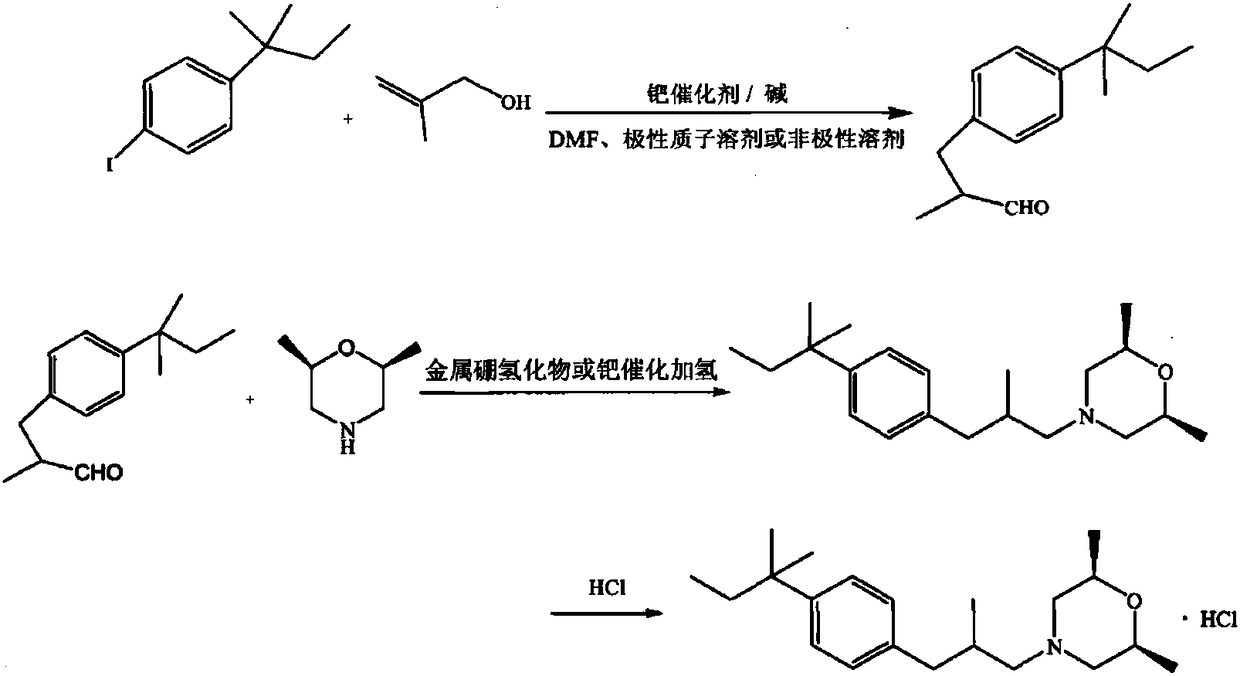
Amorolfine hydrochloride preparation method
A compound and catalyst technology, applied in the field of preparation of amorolfine hydrochloride, can solve the problems of long steps, high cost, low yield and the like, and achieve the effects of low cost, good product quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of (E)-1-((2R,6S)-2,6-dimethylmorpholine)-2-dimethyl-3-phenyl-2-en-1-one compound formula Ⅲ
[0041]
[0042] Compound formula IIa (15.00g, 0.092mol), HBTU (34.89g, 0.092mol), N,N-diisopropylethylamine (23.78g, 0.184mol) were added to N,N-dimethylacetamide (150mL), stir well. After reacting at 25°C for 0.5h, cis-2,6-dimethylmorpholine (12.73g, 0.110mol) was added and reacted at 25°C for 2-3h. Cool to room temperature, add the reaction solution into water, dichloromethane extraction and separation, and the dichloromethane phase was extracted and separation with saturated brine, the organic layer was taken, and then concentrated to dryness to obtain a yellow oil compound formula III with a yield of 95.36 %, HPLC detection purity 98.79%.
Embodiment 11
[0044] Referring to the method of Example 1, the intermediate III reaction solvent was screened and optimized, and the experimental results are shown in the table below.
[0045] serial number
Embodiment 12
[0047] Referring to the method of Example 1, the condensing agent for the reaction of intermediate III was screened and optimized, and the experimental results are shown in the table below.
[0048] serial number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com