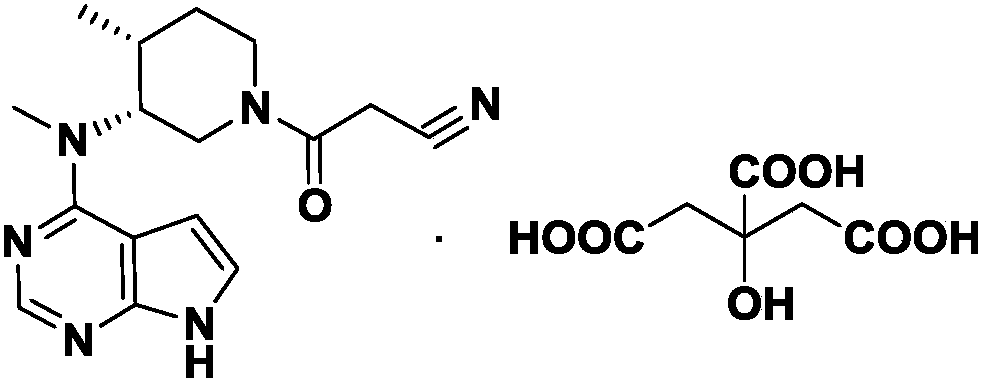
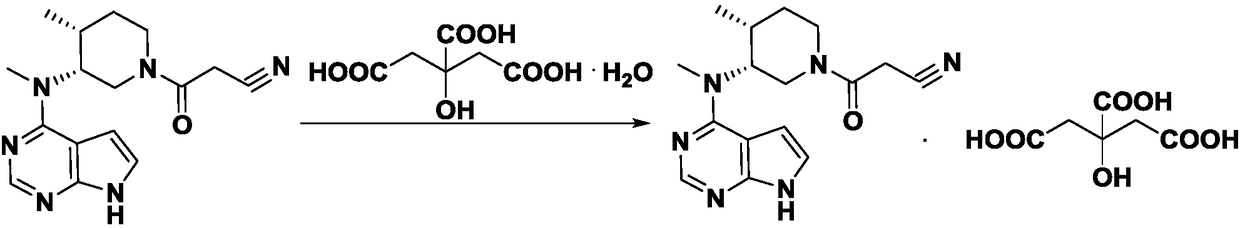
Refining method for tolfirinib citrate compound
A technology of tofacitinib and refining method, which is applied in the field of refining tofacitinib citrate compound, can solve the problems of reducing product purity and quality, low yield, etc., and achieves reduced losses, good product purity and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Tofacitinib Citrate Crude Product
[0022] Add 31.2g of tofacitinib crude product (purchased from Estel (Chengdu) Biopharmaceutical Co., Ltd.) into a 250mL reaction bottle and dissolve in 90mL of acetone / n-propanol mixed solvent (acetone / n-propanol=1.5 / 1, v / In v), heat to 75-80°C, stir to dissolve, dissolve 22.1g of citric acid monohydrate and 10mL of mixed solvent, then slowly add it dropwise into the reaction bottle, keep warm and stir for 2h, cool down to 15-20°C, Continue to stir for 1-2 hours, filter, wash the filter cake with a solvent, and dry it under vacuum at 40°C for 8 hours to obtain 47.6g, with a yield of 94.3%, a purity of 94.89% (detected by HPLC), a maximum of 1.71%, and a total of 4.35%. .
Embodiment 2
[0024] Preparation of Tofacitinib Citrate Crude Product
[0025] In a 250mL reaction bottle, 31.2g of tofacitinib crude product (purchased from Nanjing Elsa Biotechnology Co., Ltd.) was dissolved in 90mL of acetonitrile / acetone mixed solvent (acetonitrile / acetone=1 / 1, v / v), heated to 75-80°C, stir to dissolve, dissolve 31.5g citric acid monohydrate and 10mL mixed solvent, then slowly add dropwise to the reaction bottle, keep stirring for 2 hours, cool down to 15-20°C, continue stirring for 1-2 hours, After filtering, the filter cake was washed with a solvent, and dried under vacuum at 50°C for 10 h to obtain 47.2 g, with a yield of 93.7%, a purity of 94.11% (HPLC detection), a maximum single impurity of 1.92%, and a total impurity of 3.95%.
Embodiment 3
[0027] Preparation of Tofacitinib Citrate Crude Product
[0028] Add 31.2g of tofacitinib crude product (purchased from Estel (Chengdu) Biopharmaceutical Co., Ltd.) into a 250mL reaction bottle and dissolve in 90mL n-propanol / acetonitrile mixed solvent (n-propanol / acetonitrile=1 / 1, v / In v), heat to 75-80°C, stir to dissolve, dissolve 25.2g of citric acid monohydrate and 10mL of mixed solvent, then slowly add it dropwise into the reaction bottle, keep warm and stir for 1h, cool down to 15-20°C, Stirring was continued for 2 h, filtered, the filter cake was washed with a solvent, and dried under vacuum at 50° C. for 8 h to obtain 47.4 g, with a yield of 94.1%, a purity of 95.19% (by HPLC detection), a maximum single impurity of 1.63%, and a total impurity of 3.53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com