A kind of Schiff base copper complex and its preparation method and application
A technology of Schiff base copper and complexes, applied in the field of organic electroluminescent materials and their preparation, to achieve the effects of easy operation, mild conditions and simple principle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
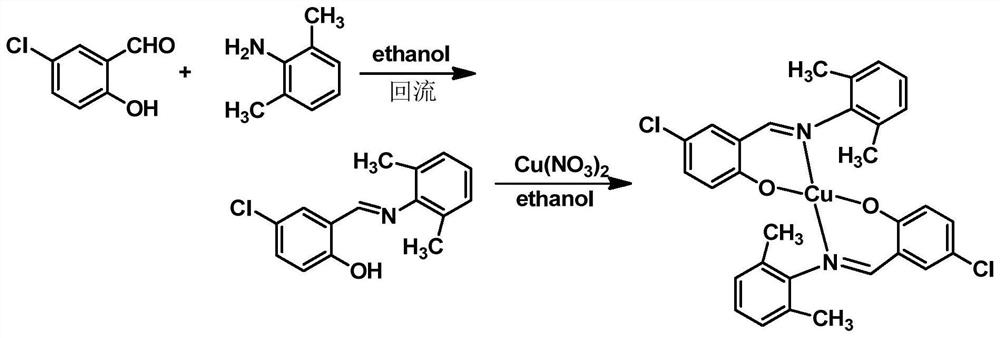
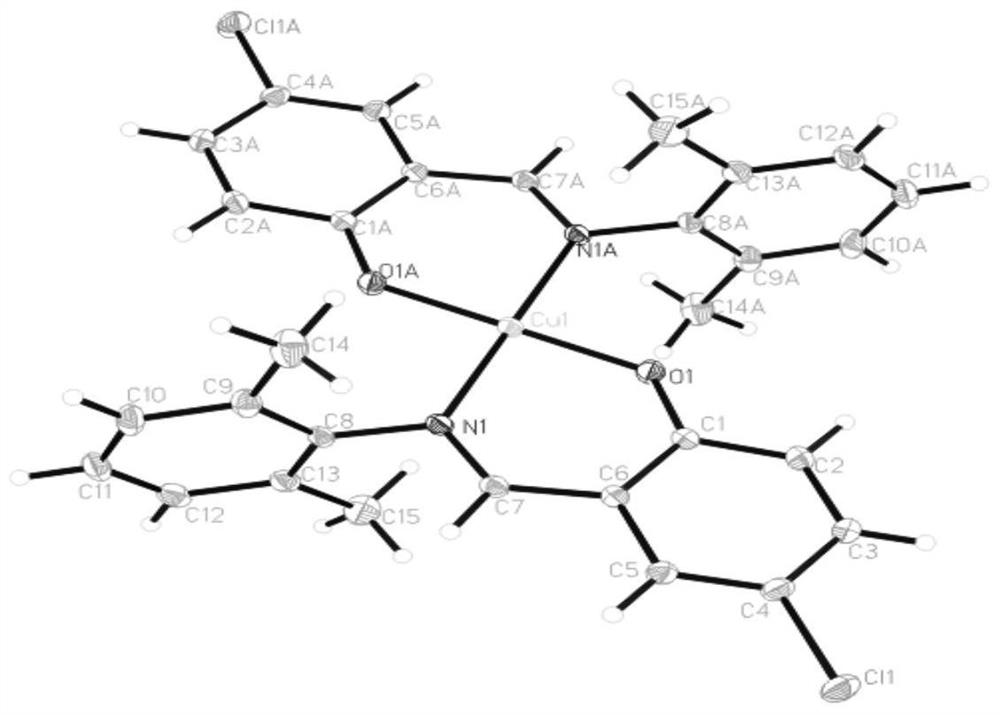
[0023] Such as figure 1 The preparation process shown in the preparation of 4-chloro-2-(2,6-dimethylanilinomethyl) copper phenolate complex, the specific steps are as follows:
[0024] (1) Synthesis of 4-chloro-2-(2,6-dimethylanilinomethyl)phenol ligand: Weigh 1.56g of 5-chlorosalicylaldehyde into a 100mL round bottom flask, add 30mL of ethanol and stir to dissolve , then add 20 mL of 1.21 g of 2,6-dimethylaniline ethanol solution, and add 0.05 mL of glacial acetic acid to obtain a reaction liquid; heat the reaction liquid to 76°C for reflux reaction for 1 h, cool to room temperature, let stand to precipitate solid, filter, And wash the solid with cold ethanol to finally obtain an orange-red solid which is 4-chloro-2-(2,6-dimethylanilinomethyl)phenol ligand;
[0025] (2) Synthesis of 4-chloro-2-(2,6-dimethylanilinomethyl) phenol copper complex: Weigh the 4-chloro-2-(2,6-dimethyl Add 0.52g of anilinomethyl)phenol ligand to a 100mL round-bottomed flask, add 30mL of ethanol and...
Embodiment 2
[0027] Such as figure 1 The preparation process shown in the preparation of 4-chloro-2-(2,6-dimethylanilinomethyl) copper phenolate complex, the specific steps are as follows:
[0028] (1) Synthesis of 4-chloro-2-(2,6-dimethylanilinomethyl)phenol ligand: Weigh 1.56g of 5-chlorosalicylaldehyde into a 100mL round bottom flask, add 30mL of ethanol and stir to dissolve , then add 20 mL of 1.21 g of 2,6-dimethylaniline ethanol solution, and add 0.1 mL of glacial acetic acid to obtain a reaction liquid; heat the reaction liquid to 78 °C for reflux reaction for 1 h, cool to room temperature, let stand to precipitate solid, filter, And wash the solid with cold ethanol to finally obtain an orange-red solid which is 4-chloro-2-(2,6-dimethylanilinomethyl)phenol ligand;
[0029] (2) Synthesis of 4-chloro-2-(2,6-dimethylanilinomethyl) phenol copper complex: Weigh the 4-chloro-2-(2,6-dimethyl Add 0.52g of anilinomethyl)phenol ligand to a 100mL round bottom flask, add 30mL of ethanol and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com