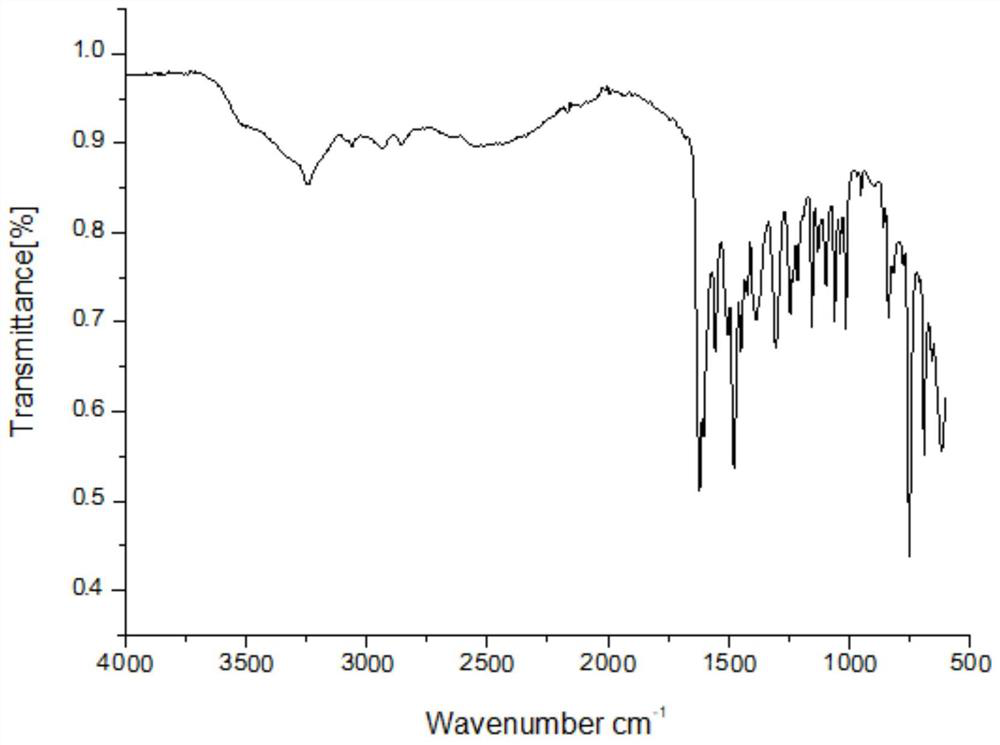
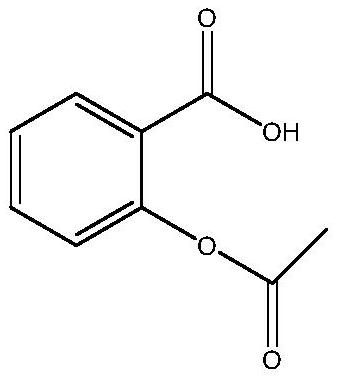
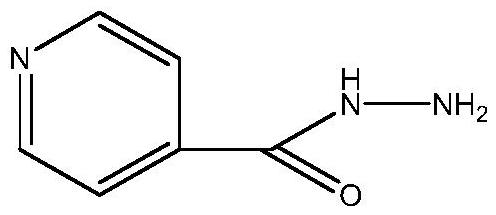
Acetylsalicylic acid-isoniazid hybrid compound and its preparation method and application
A technology of acetylsalicylic acid and isoniazid, applied in the direction of organic chemistry, antibacterial drugs, etc., can solve the problem of not finding acetylsalicylic acid-isoniazid hybrid drugs, etc., to prevent the drug resistance of Mycobacterium tuberculosis , Good application prospect, strong activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 1.31g of acetylsalicylic acid and 1g of isoniazid into a 50mL single-necked flask, then add 30mL of DMF to dissolve completely, then add 1.5g of DCC and 0.05g of DMAP into the single-necked flask and stir evenly. The one-necked flask was placed on a collector-type constant-temperature heating magnetic stirrer, the temperature was raised to 30° C. and the reaction was stirred at a constant temperature, and the reaction was tracked with a thin-layer chromatography silica gel plate. After the reaction is finished, filter with a sand core funnel, slowly add the filtrate dropwise to 240 mL of ice water, and stir while adding. The obtained mixture was suction filtered again with a sand core funnel, and the filter cake was washed 1-3 times with ice water, and then washed once with saturated NaCl solution. Finally, the filter cake was transferred to a vacuum desiccator for sufficient drying to obtain 1.4 g of pure acetylsalicylic acid-isoniazid hybrid drug. Analysis and ca...
Embodiment 2
[0030] Add 1.31g of acetylsalicylic acid and 1g of isoniazid into a 50mL single-necked flask, then add 30mL of DMF to dissolve completely, then add 1.5g of DCC and 0.05g of DMAP into the single-necked flask and stir evenly. The one-necked flask was placed on a collector-type constant temperature heating magnetic stirrer, the temperature was raised to 27° C. and the reaction was stirred at a constant temperature, and the reaction was tracked with a thin-layer chromatography silica gel plate. After the reaction is finished, filter with a sand core funnel, slowly add the filtrate dropwise to 220 mL of ice water, and stir while adding. The obtained mixture was suction filtered again with a sand core funnel, and the filter cake was washed 1-3 times with ice water, and then washed once with saturated NaCl solution. Finally, the filter cake was transferred to a vacuum dryer to fully dry to obtain pure acetylsalicylic acid-isoniazid. Through analysis and calculation, the content of a...
Embodiment 3
[0032] Add 1.31g of acetylsalicylic acid and 1g of isoniazid into a 50mL single-necked flask, then add 30mL of DMF to dissolve completely, then add 1.5g of DCC and 0.05g of DMAP into the single-necked flask and stir evenly. The one-necked flask was placed on a collector-type constant-temperature heating magnetic stirrer, the temperature was raised to 33° C. and the reaction was stirred at a constant temperature, and the reaction was tracked with a thin-layer chromatography silica gel plate. After the reaction is finished, filter with a sand core funnel, slowly add the filtrate dropwise to 260 mL of ice water, and stir while adding. The obtained mixture was suction filtered again with a sand core funnel, and the filter cake was washed 1-3 times with ice water, and then washed once with saturated NaCl solution. Finally, the filter cake was transferred to a vacuum dryer to fully dry to obtain pure acetylsalicylic acid-isoniazid. Through analysis and calculation, the content of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com