Application of fast green in inhibition of aggregating of beta-amyloid protein
A kind of amyloid, application technology, applied in health products or food, fast green as β-amyloid aggregation inhibitor in the field of medicine, can solve the problem that the process of inhibiting aggregation is not easy to control, and achieve the purpose of inhibiting β-amyloid Aggregation, slowing down the process of conversion, inhibiting the effect of good effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
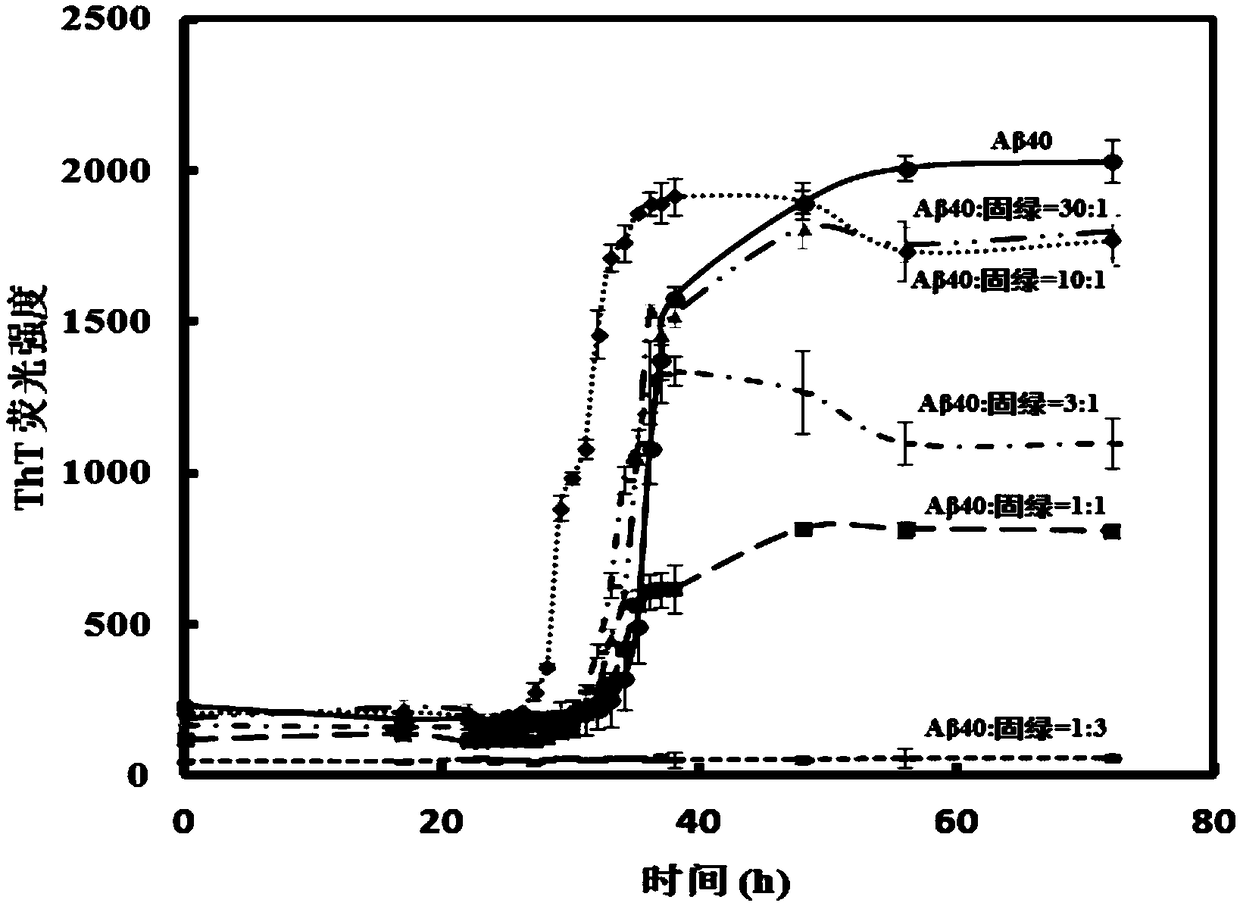
[0041] Example 1 : Changes in fluorescence intensity of Thioflavin (ThT) after co-cultivation with different concentrations of Fast Green and Aβ40 for different time
[0042]First, Aβ40 was dissolved in hexafluoroisopropanol solution to obtain a 1 mg / mL Aβ40 solution, sonicated for 10 minutes to make Aβ40 in a monodisperse state, freeze-dried to obtain Aβ40 dry powder, and stored at -20°C. Then, 0.6 mg of Aβ40 powder was weighed, dissolved in 0.4 mL of 20 mM NaOH solution to a final concentration of 330 μM, and sonicated for 10 min to fully dissolve it to obtain an Aβ40 mother solution with a concentration of 330 μM.
[0043] Next, 10.512mg ThT was weighed and dissolved in 100mL phosphate buffer saline (PBS, phosphate buffersaline, wherein the concentration of phosphate was 100mM, and the concentration of NaCl was 10mM), to obtain a ThT mother solution with a concentration of 330μM.
[0044] Weigh 16,000 g of Aβ40 mother solution with a concentration of 330 μM and centrifuge...
Embodiment 2
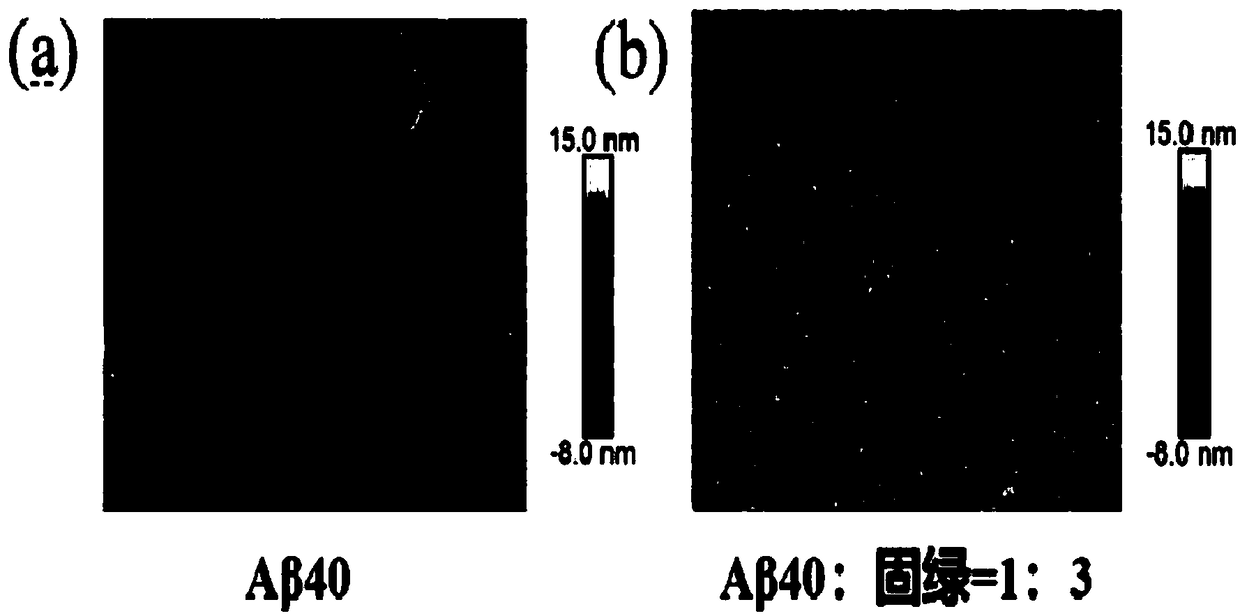
[0049] Example 2 : Aggregation morphology changes of cultures after co-cultivation of Fast Green and Aβ40 for different time
[0050] Aβ40 was treated in the same manner as in Example 1, and an Aβ40 solution containing 90 μM fast green was prepared, and the final concentration of Aβ40 in the solution was 30 μM, that is, the ratio of Aβ40:fast green was 1:3. The above solution was incubated in a microplate reader at 37°C and shaken for 5 s every 10 min.
[0051] After culturing for 48 hours, take 100 μL of Aβ40 culture solution and ultrasonicate for 10 minutes; fix the circular iron sheet with double-sided adhesive tape in a clean petri dish, then fix the mica sheet on the circular iron sheet, stick the mica sheet three times continuously with scotch tape, remove Unclean layer of mica flakes. Use a 20 μL pipette gun to take 20 μL of the ultrasonically prepared sample and drop it on the mica sheet. After 5-10 minutes, use a pipette gun to take 200 μL Wahaha pure water and rin...
Embodiment 3
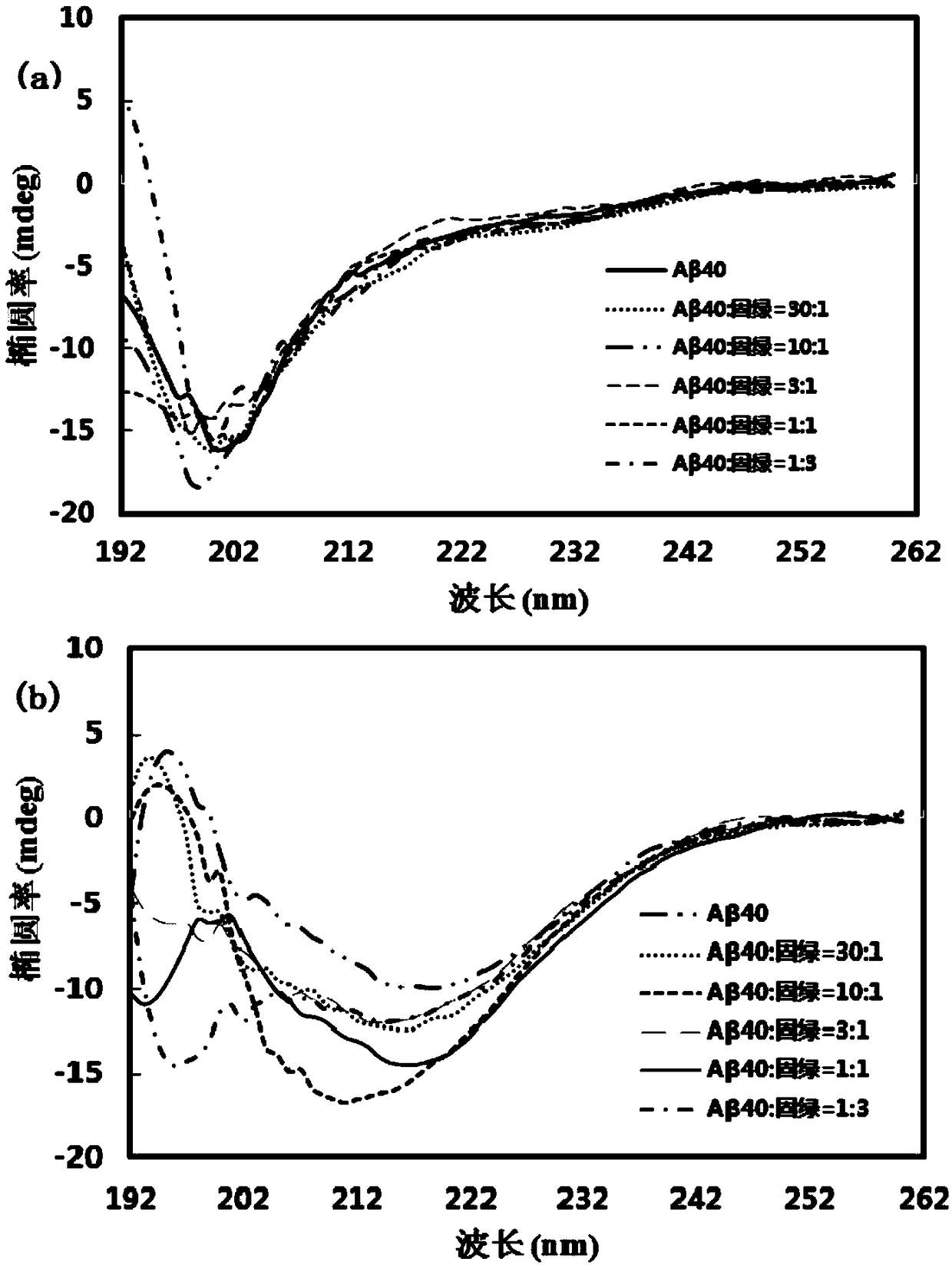
[0053] Example 3: Changes in the secondary structure of cultures after co-cultivation with different concentrations of Fast Green and Aβ40 for different time
[0054] Aβ40 was treated according to the same method as in Example 1, and Aβ40 culture solutions containing different fast green concentrations (1 μM, 3 μM, 10 μM, 30 μM, 90 μM) were prepared, wherein the final concentration of Aβ40 was 30 μM, that is, the concentration of Aβ40 and fast green in the solution The concentration ratios were 30:1, 10:1, 3:1, 1:1, 1:3, and the above solutions were cultured at 37°C and 140rpm.
[0055] After culturing for 48 hours, take 400 μL of the culture solution and add it to a CD detection cell with an optical path of 1 mm for detection. The wavelength scanning range is 190-260 nm, the bandwidth is 2 nm, and the scanning speed is 100 nm / min. The experimental results are the average of three scans. The results are as follows: image 3 shown. Depend on image 3 It can be seen that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com