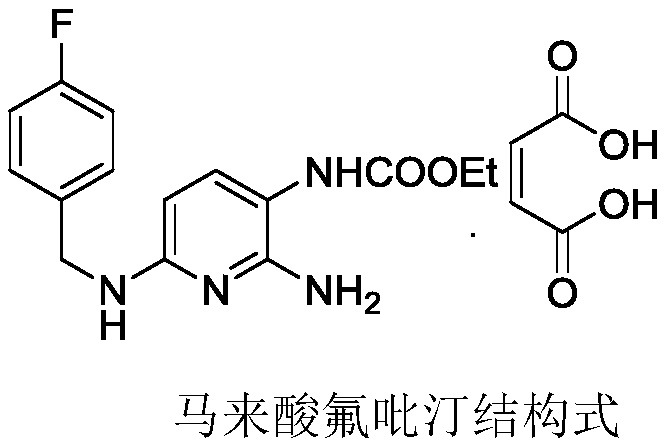
Method for preparing polymorph-A flupirtine maleate with high bulk density
A technology of flupirtine maleate and flupirtine, applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of inability to obtain high bulk density A crystal form, unable to meet preparation requirements, and low bulk density of A crystal, Achieve the effect of stable crystal content, suitable for industrial production and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
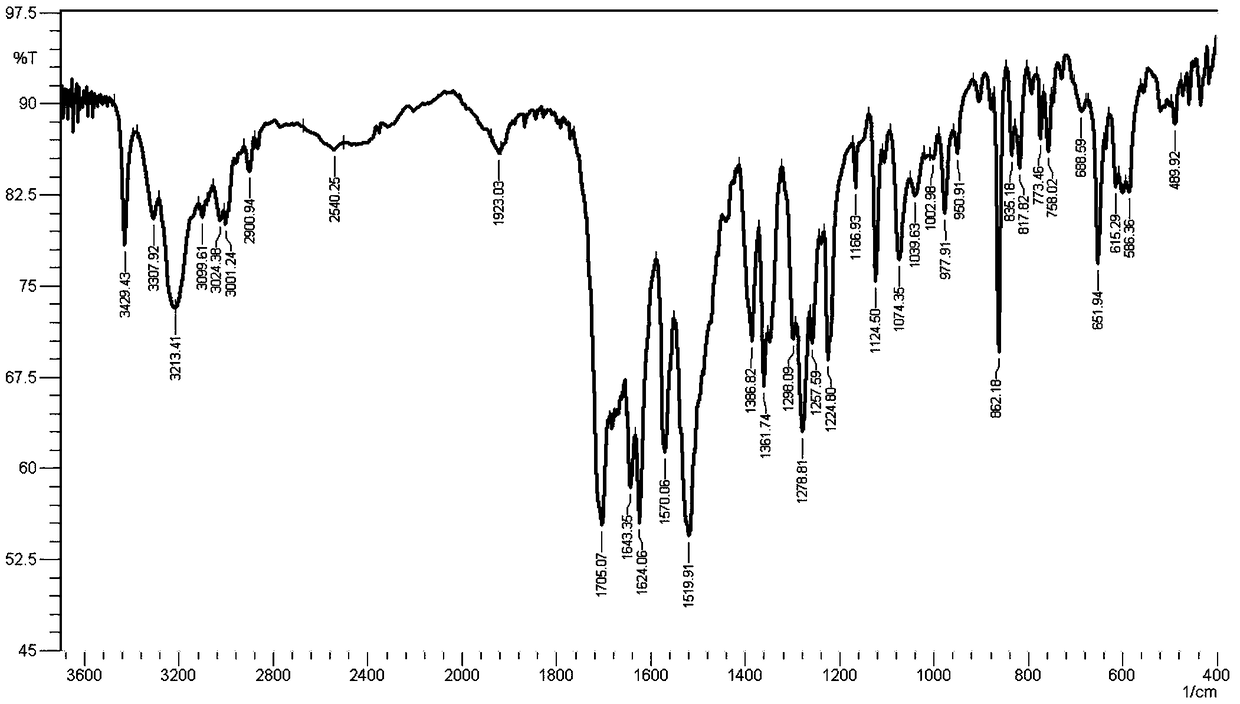
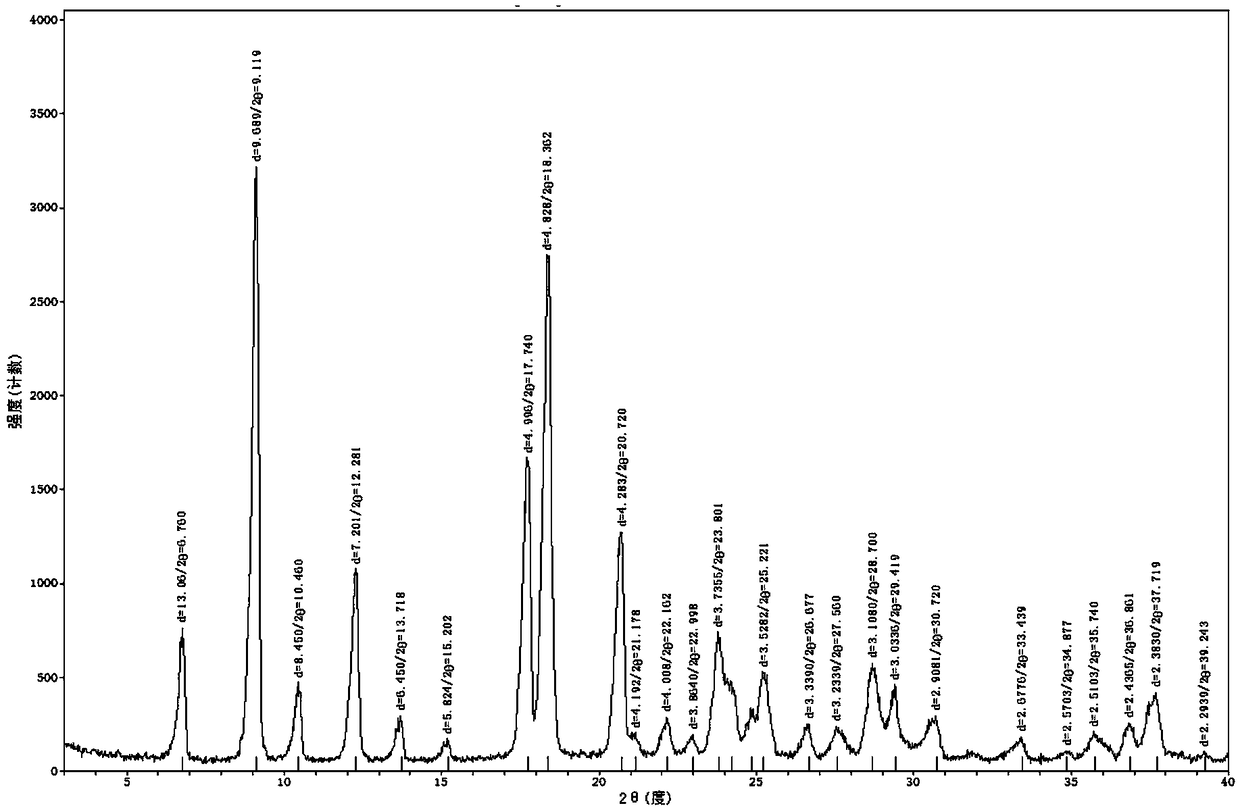
[0036] Under the protection of nitrogen, add 10g of flupirtine white gray refined product into a 1000ml three-necked flask, add 250g of isopropanol, 10g of DMSO, heat the inner temperature to 30°C, stir it mechanically, and dissolve it at a speed of 200rpm / min. Add 4.0g of horse Dissolve the acid with 20.0g of isopropanol and add it dropwise into a three-necked flask, react for 1h, filter, rinse with a small amount of isopropanol, and drain to obtain white crystals, which are then vacuum-dried to obtain pure flupirtine maleate 13.1 g, yield 95%. It was confirmed as pure A crystal form by X powder diffraction.
[0037] The diffraction angle, interplanar spacing and relative intensity of crystal A are shown in the following table:
[0038]
[0039] 1 HNMR (500MHz, DMSO-d6) δ:
[0040] 8.342(s,1H), 7-8(m,5H), 6.152(s,2H), 5.79(d,lH), 4.385(s,2H), 4.03(t,2H), 1.193(m,3H) .
[0041] A crystal infrared spectrum at 1170cm -1 There is an absorption peak nearby, and the B crys...
Embodiment 2
[0044] Under the protection of nitrogen, add 20g of flupirtine white gray refined product into a 2000ml three-necked flask, add 830g of propanol and 40g of DMSO, heat the inner temperature to 50°C, stir and dissolve by mechanical stirring at a speed of 400rpm / min, and add 9.1g of Malay The acid was dissolved in 50.0 g of propanol and added dropwise into a three-necked flask, reacted for 1 hour, filtered, rinsed with a small amount of propanol, and dried to obtain white crystals, which were dried in vacuum to obtain 26.2 g of pure flupirtine maleate. The rate is 95%. It was confirmed as pure A crystal form by X powder diffraction.
[0045] X powder diffraction, 1 HNMR and infrared spectrum are consistent with embodiment 1.
Embodiment 3
[0047] Under the protection of nitrogen, add 30g of flupirtine white gray refined product into a 2000ml three-necked flask, add 1200g of acetonitrile, 60g of DMSO, heat the inner temperature to 35°C, stir and dissolve with a speed of 250rpm / min, and dissolve 12.0g of maleic acid Dissolved with 90.0g of acetonitrile and added dropwise into a three-necked flask, reacted for 1h, filtered, rinsed with a small amount of acetonitrile, and dried to obtain white crystals, dried in vacuum to obtain 39.7g of pure flupirtine maleate, with a yield of 96% . It was confirmed as pure A crystal form by X powder diffraction.
[0048] X powder diffraction, 1 HNMR and infrared spectrum are consistent with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com