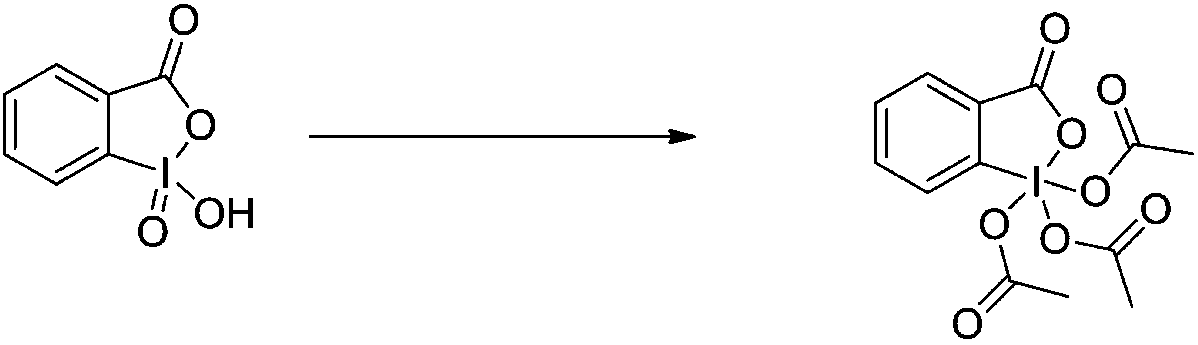
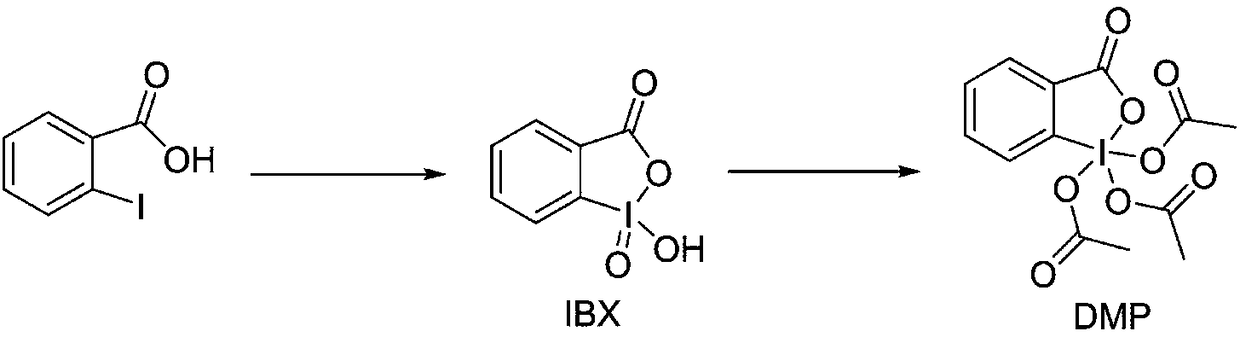
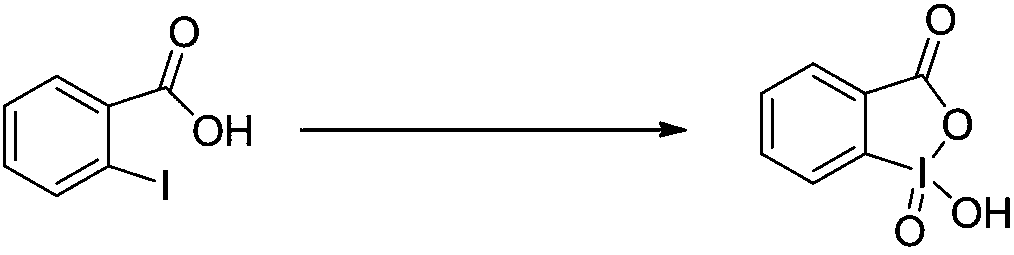Preparation method of DMP (Dess-Martin periodinane)
A technology of oxidizing agent and high boiling point solvent is applied in the field of pharmaceutical chemical synthesis, which can solve the problems of reducing the amount of acetic anhydride, low yield, etc., and achieve the effects of simple and stable operation, high purity and easy product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 2-Iodobenzoic acid (50.0 g, 0.20 mol) was added to sulfolane (250 mL), and the solution was heated to 50°C. 90% of the above solution was slowly added dropwise to an aqueous solution (300 mL) of potassium hydrogen persulfate (147.4 g, 0.24 mol, 1.2 eq) at 50°C, and the reaction was incubated for 3 hours. Then the reaction solution was heated to 95° C. and the remaining 10% 2-iodobenzoic acid solution was slowly added dropwise to the reaction solution, and the reaction was continued for 3 hours. After the reaction was complete, it was cooled to 5°C and stirring was continued at this temperature for 1.5 hours. Add 1L of water under stirring, filter (the filtrate is to be recycled), the filter cake is rinsed with water and acetone successively, and dried at room temperature for 16 hours to obtain 53.2 g of 2-iodoxybenzoic acid (yield 95.04%), a white solid, and the purity of IBX is 99.7 % (confirmed by NMR and HPLC). The solid was determined to be IBX by HNMR detection. ...
Embodiment 2
[0040] 2-Iodobenzoic acid (5kg, 20mol) was added to DMF (25L), and the solution was heated to 60°C. 85% of the above solution was slowly added dropwise to an aqueous solution (40L) of potassium hydrogen persulfate (22.1kg, 36mol, 1.8eq) at 60°C, and the reaction was incubated for 3 hours. The reaction was heated to 95° C. and the remaining 15% 2-iodobenzoic acid solution was slowly added dropwise to the reaction liquid, and the reaction was continued for 2 hours. After the reaction was complete it was cooled to 0°C and stirring was continued at this temperature for 1 hour. 100L of water was added under stirring, and after filtration, the filter cake was rinsed with water and acetone successively and dried at room temperature for 16 hours to obtain 4.51kg (yield 80.53%) of a white solid, and the purity of IBX was 99.8% (confirmed by NMR and HPLC). The standard spectrum is consistent.
[0041] Add IBX (4.5kg, 16mol) into a mixed solvent of acetic anhydride (5.88kg, 57.6mol) an...
Embodiment 3
[0043] 2-Iodobenzoic acid (50kg, 200mol) was added to dimethylsulfoxide (250L), and the solution was heated to 55°C. 90% of the above solution was slowly added dropwise to an aqueous solution (300 L) of potassium hydrogen persulfate (159.6 kg, 260 mol, 1.3 equiv) at 70°C. React at 70°C for 3 hours. The reaction was heated to 90° C., and the remaining 10% 2-iodobenzoic acid solution was slowly added dropwise to the reaction liquid, and the reaction was continued for 3 hours. After the reaction was complete it was cooled to 0°C and stirring was continued at this temperature for 2 hours. 1000L of water was added under stirring, and the filtered cake was rinsed with water and acetone successively and dried at room temperature for 16 hours to obtain 45.4kg of white solid (yield 81.07%). The purity of IBX was 99.8% (confirmed by NMR and HPLC). NMR and standard The spectra are consistent.
[0044] 2-iodylbenzoic acid (45kg, 160mol) was added to acetic anhydride (53.8kg, 528mol) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com