A kind of rhein ester derivative and its preparation method and application
A technology of rhein esters and derivatives, applied in chemical instruments and methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem of low bioavailability, limited application of rhein, and not particularly ideal solubility question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
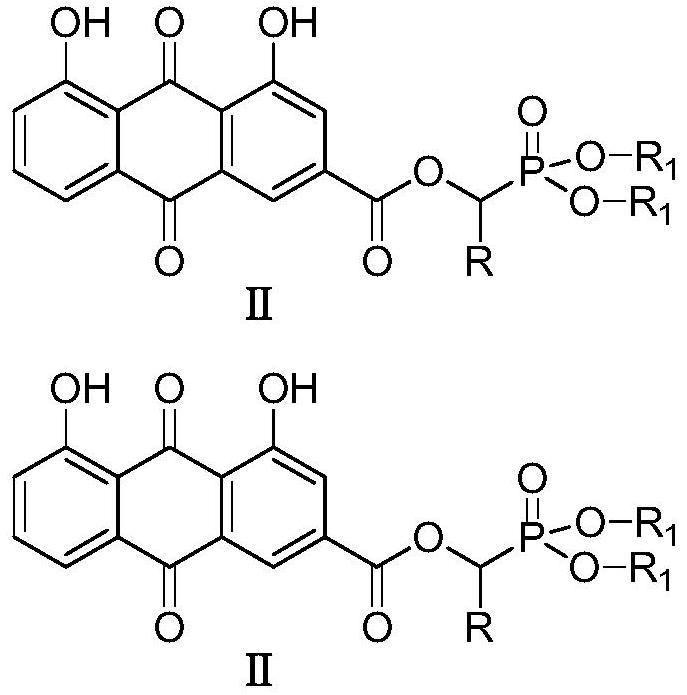
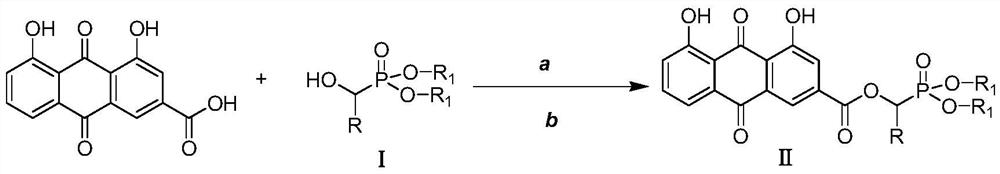
[0023] Preparation of O',O-Dimethyl-α-rheinyl-α-phenyl-phosphonic acid methyl ester (Ⅱa)
[0024] 2.0mmol rhein, 2.2mmol intermediate I (dimethyl α-hydroxybenzylphosphonate), and 1.0mmol [Bmim] BF 4 Add the ionic liquid into the three-necked flask, then add 2.0mmol DCC and 1.6mmol DMAP to the three-necked flask at 0-5°C, and finally add 40mL of anhydrous dichloromethane into the reaction flask, put it into an ultrasonic synthesizer, and set it at a power of 100W , naturally warming up to 20° C., maintaining the temperature and stirring for 20 minutes. After completion, filter and concentrate under reduced pressure to obtain a crude product, which is separated and purified by column chromatography, and finally the target product is obtained as a light yellow solid (IIa), with a yield of 84.3%. compound 1 H NMR and 13 C NMR: 1 H-NMR (400MHz, CDCl 3 )δ:12.08(s,1H,OH),11.99(s,1H,OH),7.80-7.85(m,2H,ArH),7.63(s,1H,ArH),7.22-7.40(m,7H,ArH ),6.55(s,1H,PCH),3.32-3.40(m,6H,2OCH 3 ...
Embodiment 2
[0026] Preparation of O',O-diethyl-α-rheinyl-α-o-fluorophenyl-phosphonic acid methyl ester (Ⅱb)
[0027] 2.0mmol of rhein, 2.2mmol of intermediate Ⅰ (diethyl α-hydroxy-o-fluorobenzylphosphonate), and 1.0mmol of [Bmim] BF 4 Add the ionic liquid into the three-necked flask, then add 2.0mmol DCC and 1.6mmol DMAP into the three-necked flask at 0-5°C, and finally add 60mL of anhydrous dichloromethane into the reaction flask, put it into an ultrasonic synthesizer, and set it at a power of 100W , naturally warming up to 25°C, maintaining the temperature and stirring the reaction for 15min, complete, filter, and concentrate under reduced pressure to obtain the crude product, which is separated and purified by column chromatography, and finally the target product is obtained as a light yellow solid (Ⅱa), with a yield of 82.6%. compound 1 H NMR and 13 C NMR: 1 H-NMR (400MHz, CDCl 3 )δ:12.08(s,1H,OH),11.98(s,1H,OH),7.84-7.90(m,2H,ArH),7.69-7.73(s,1H,ArH),7.24-7.40(m,6H ,ArH),6.58(s,...
Embodiment 3
[0029] Preparation of O',O-diethyl-α-rheinyl-α-(2-furyl)-phosphonic acid methyl ester (Ⅱc)
[0030] 2.0mmol rhein, 2.2mmol intermediate Ⅰ [α-hydroxy-(2-furylmethyl) phosphonic acid diethyl ester], and 1.0mmol [Pmim] [HSO 4 ] Add the ionic liquid into the three-necked flask, then add 2.0mmol DCC and 1.6mmol DMAP to the three-necked flask at 0-5°C, and finally add 100mL of anhydrous dichloromethane into the reaction flask, put it into the ultrasonic synthesizer, and 80W, naturally warming up to 20°C, maintaining the temperature and stirring for 30min, after completion, filtering and concentrating under reduced pressure to obtain the crude product, which was separated and purified by column chromatography, and finally the target product was obtained as a light yellow solid (Ⅱa), with a yield of 85.0% . compound 1 H NMR and 13 C NMR: 1H-NMR (400MHz, CDCl 3 )δ:12.08(s,1H,OH),11.97(s,1H,OH),7.88-8.01(m,2H,ArH),7.12-7.35(m,4H,ArH),6.72-6.87(m,2H ,ArH),6.44(s,1H,PCH),3.96-4.05(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com