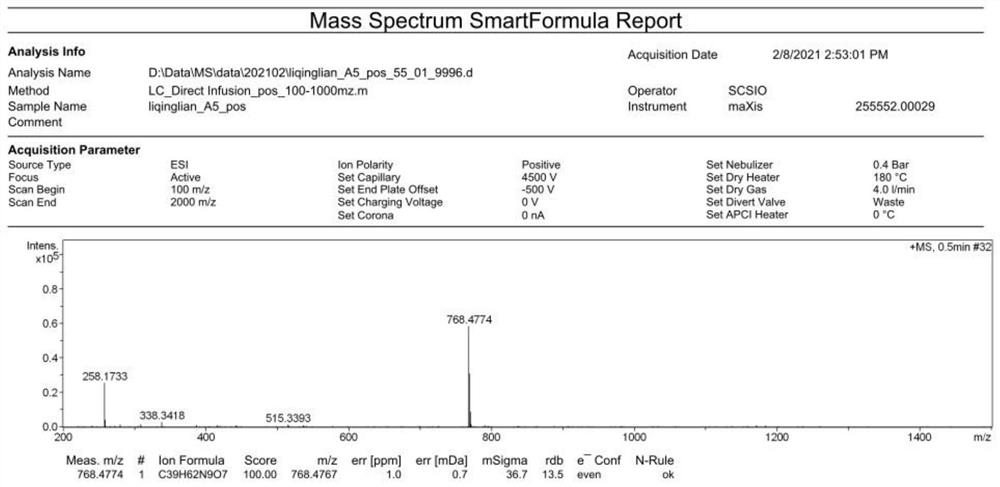
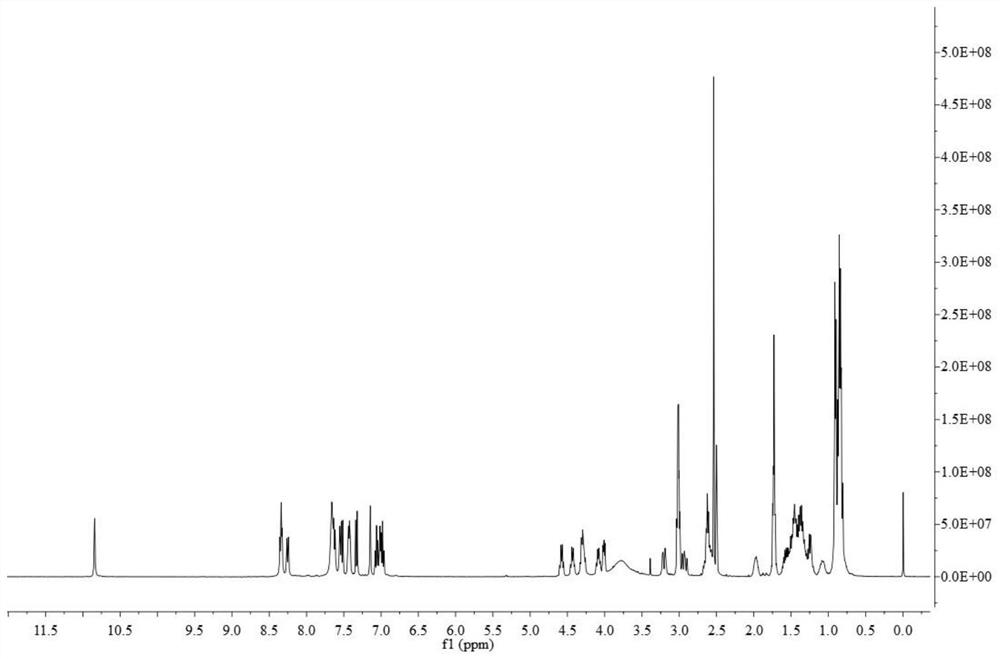
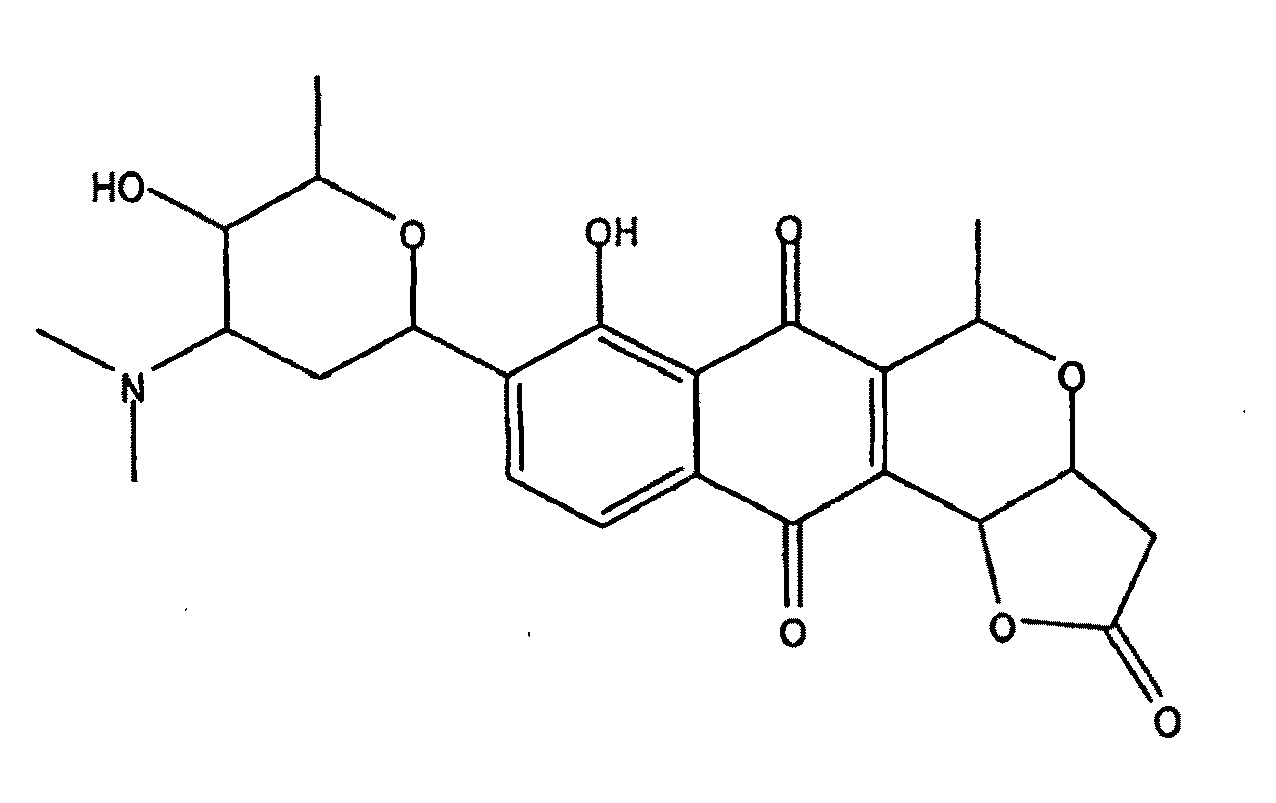
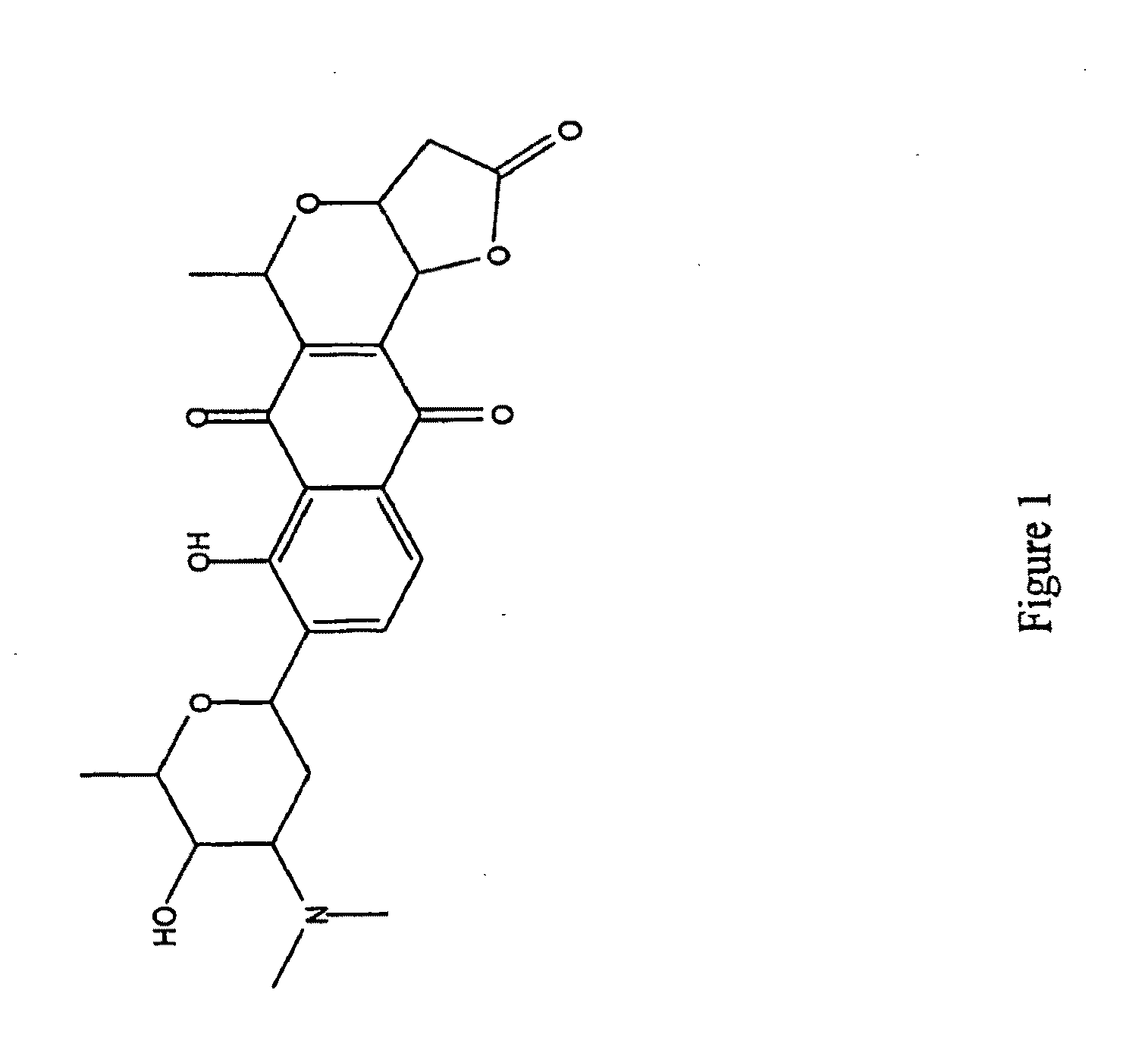
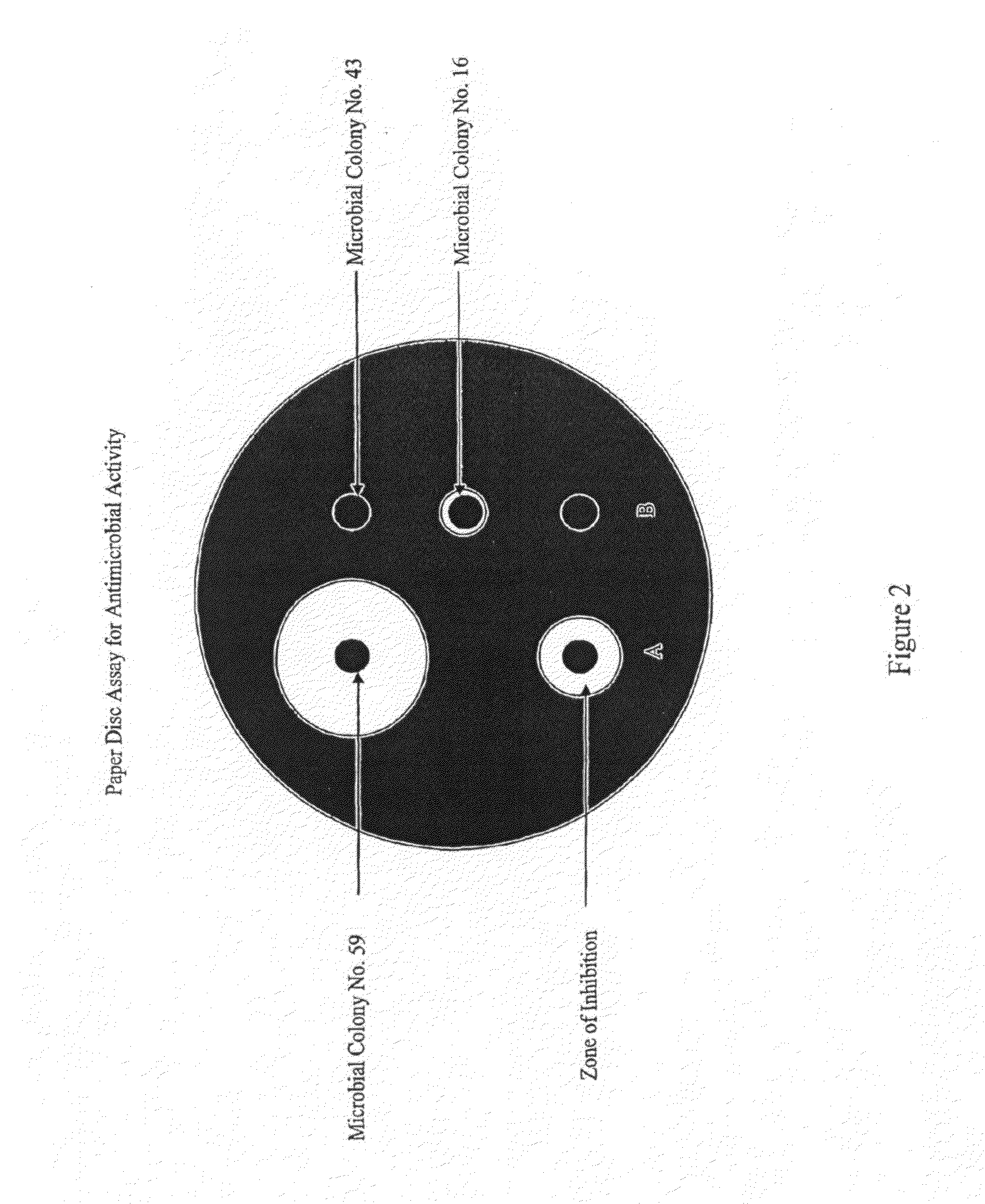
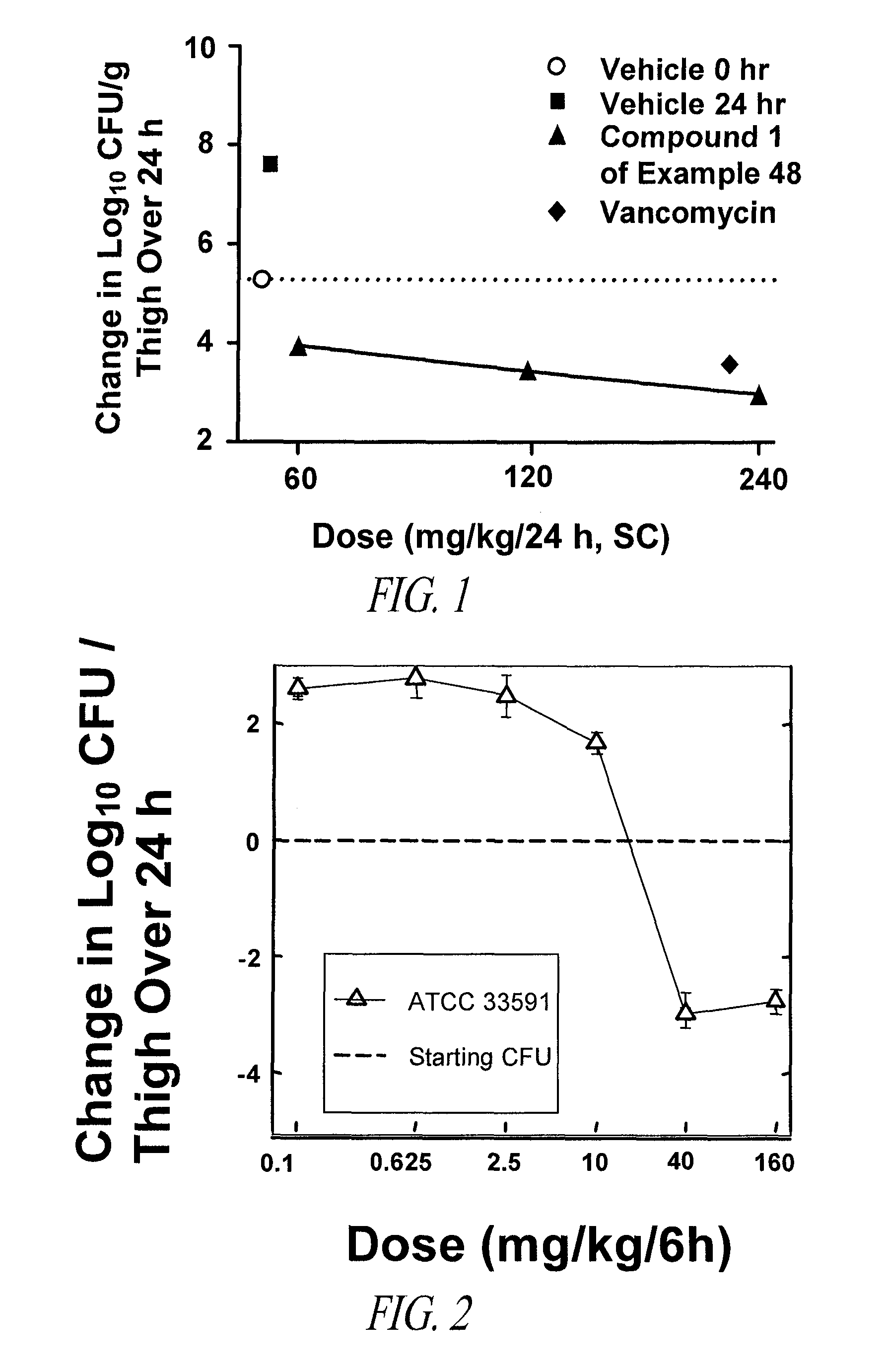
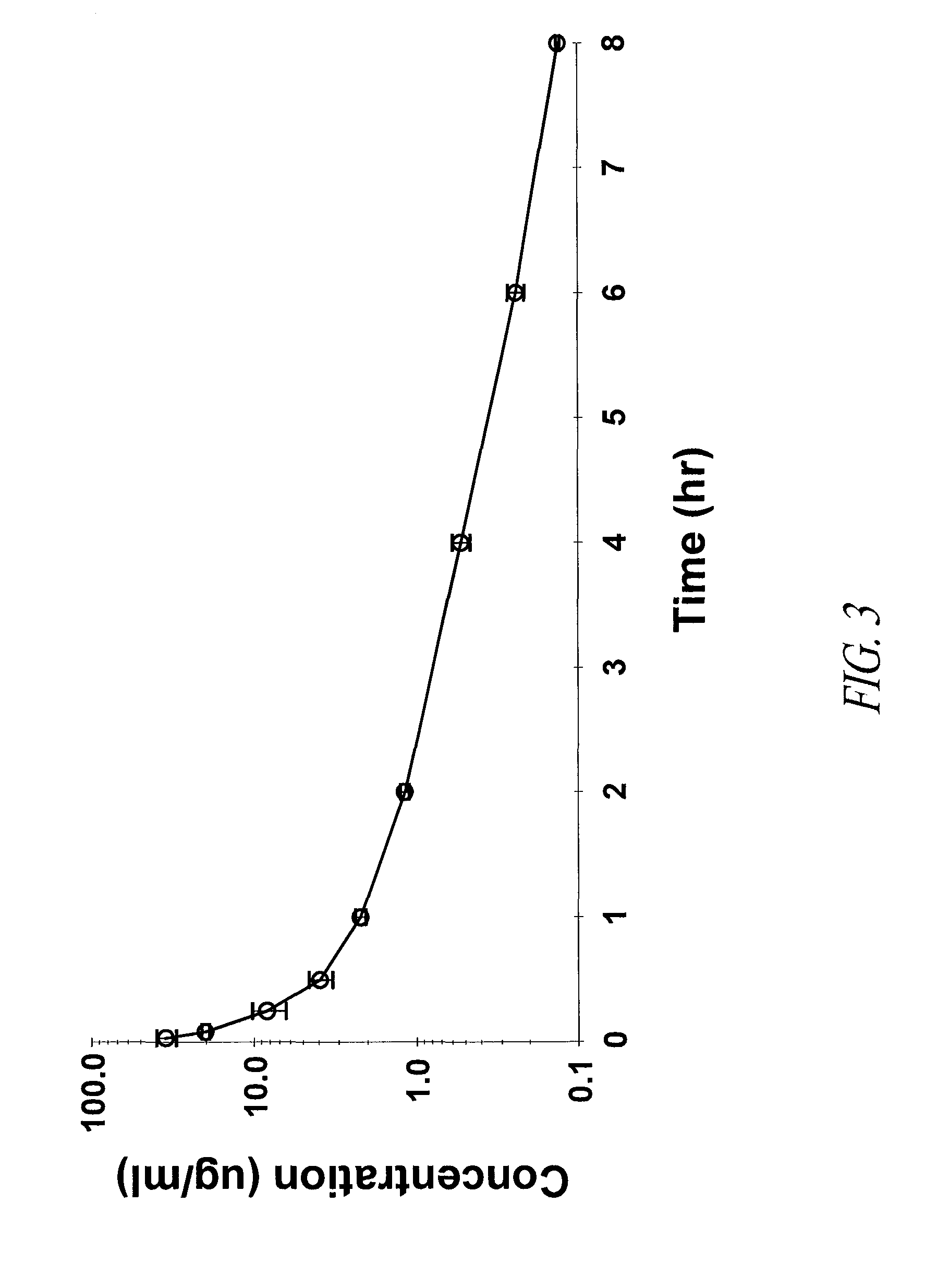
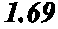Patents
Literature
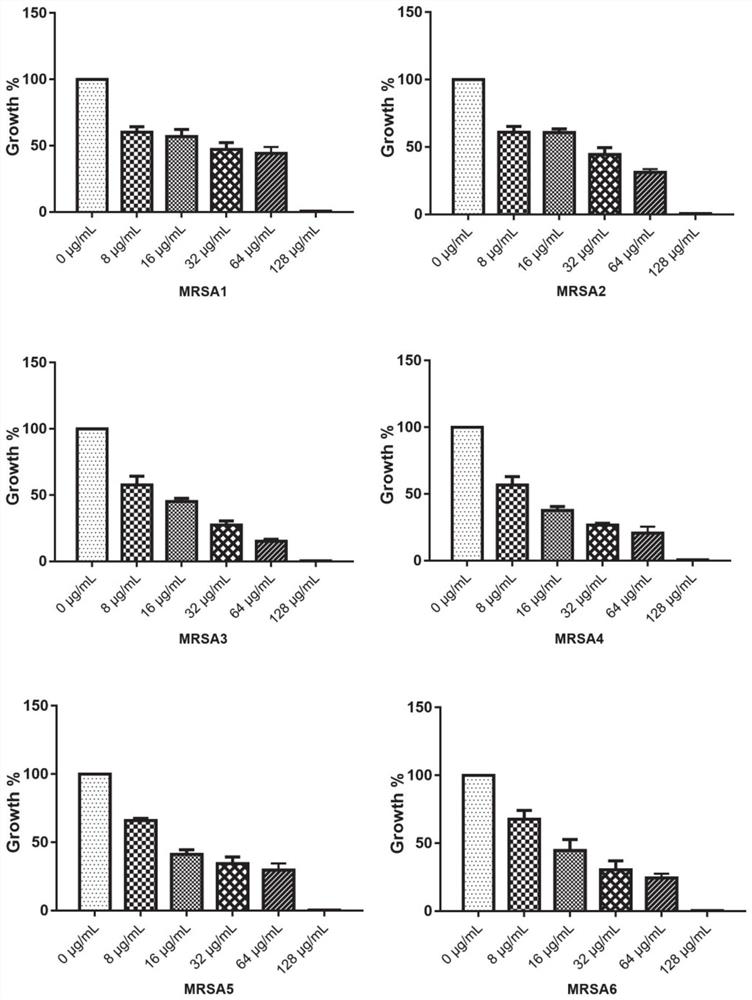
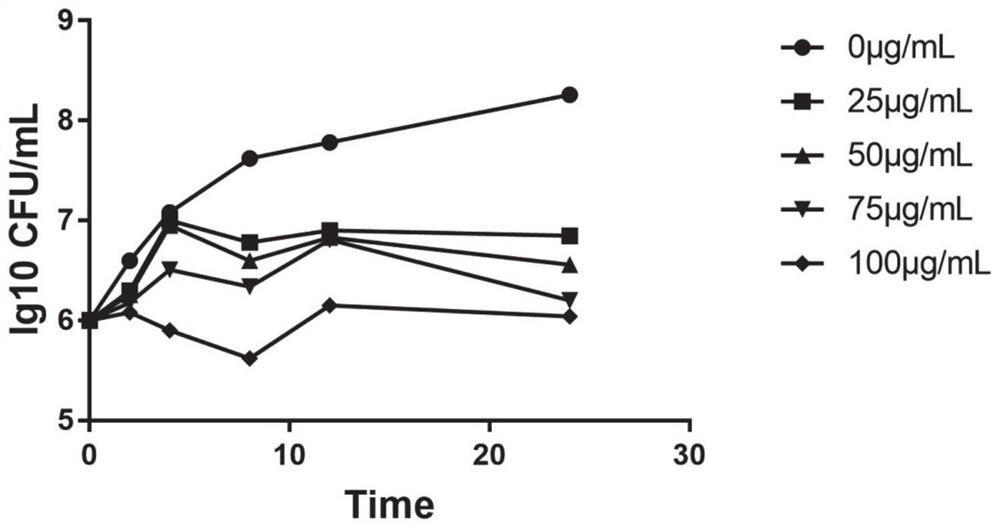
53 results about "Meticillin resistance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbacephem beta-lactam antibiotics
InactiveUS20050004095A1Antibacterial agentsOrganic active ingredientsAntibiotics beta lactamBeta lactam antibiotics
The present invention relates to carbocephem antibiotics and pharmaceutically acceptable salts thereof useful for the treatment of bacterial infections, in particular infections caused by methicillin-resistant Staphylococcus spp. bacteria.
Owner:TRINE PHARMA
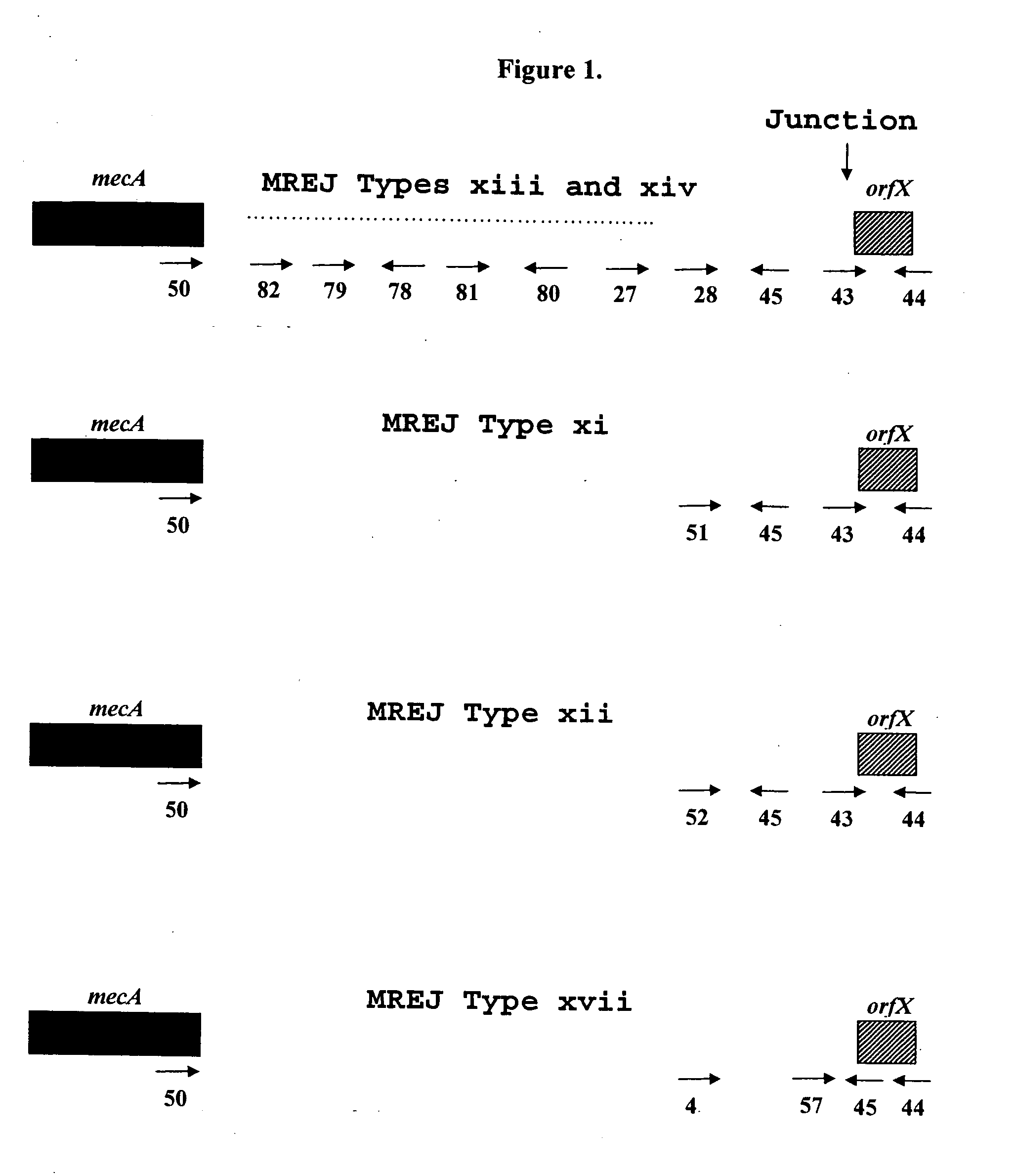
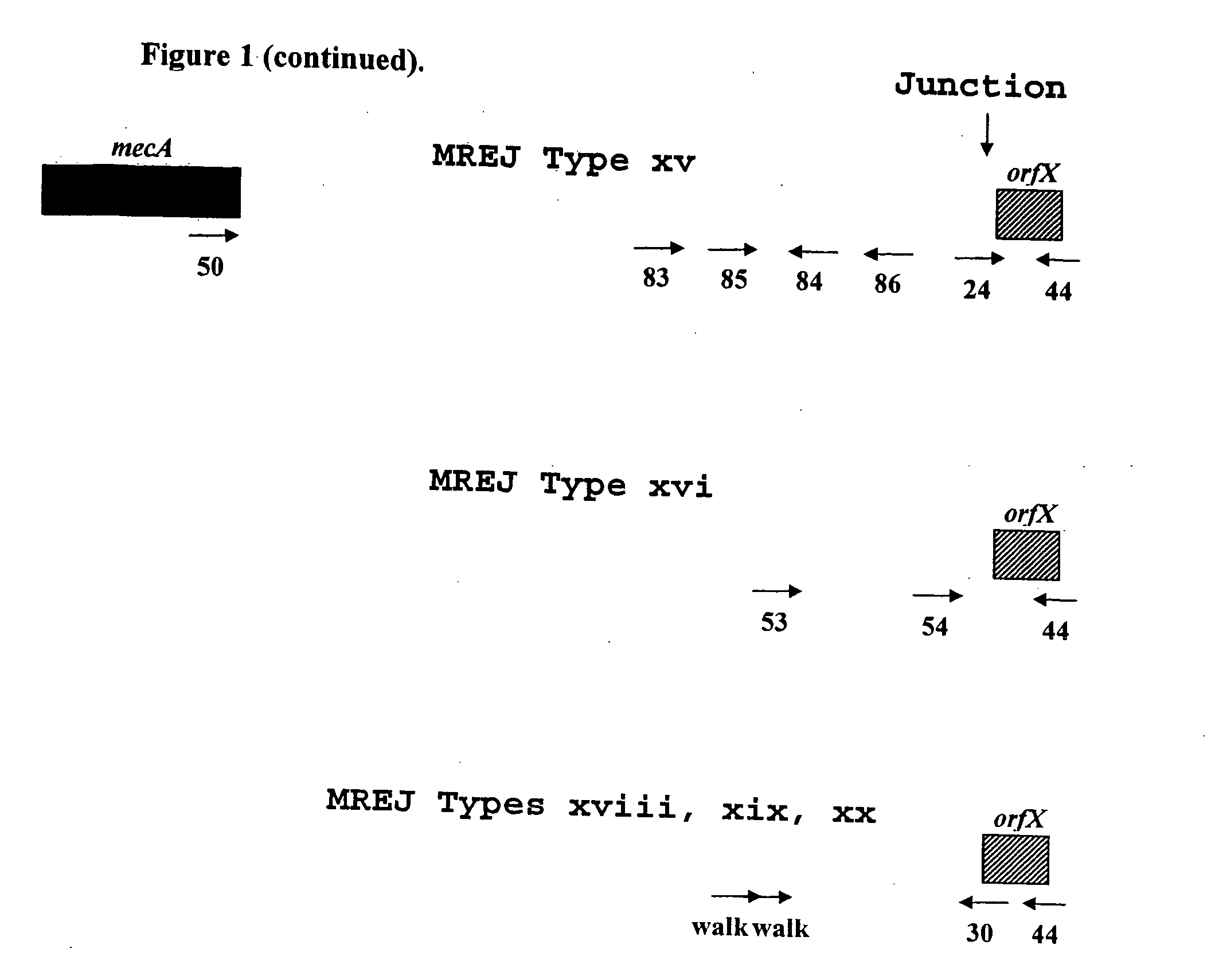
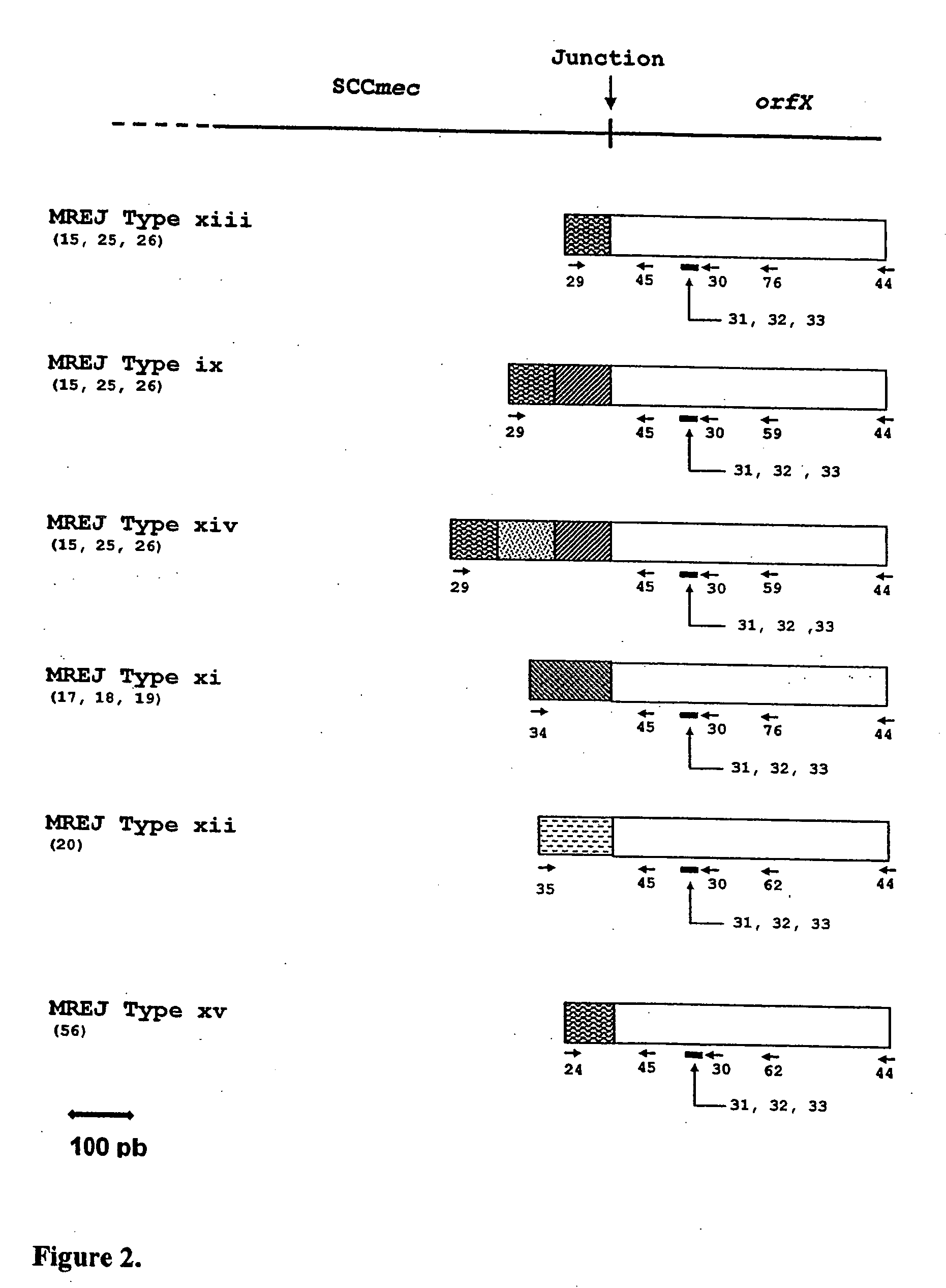
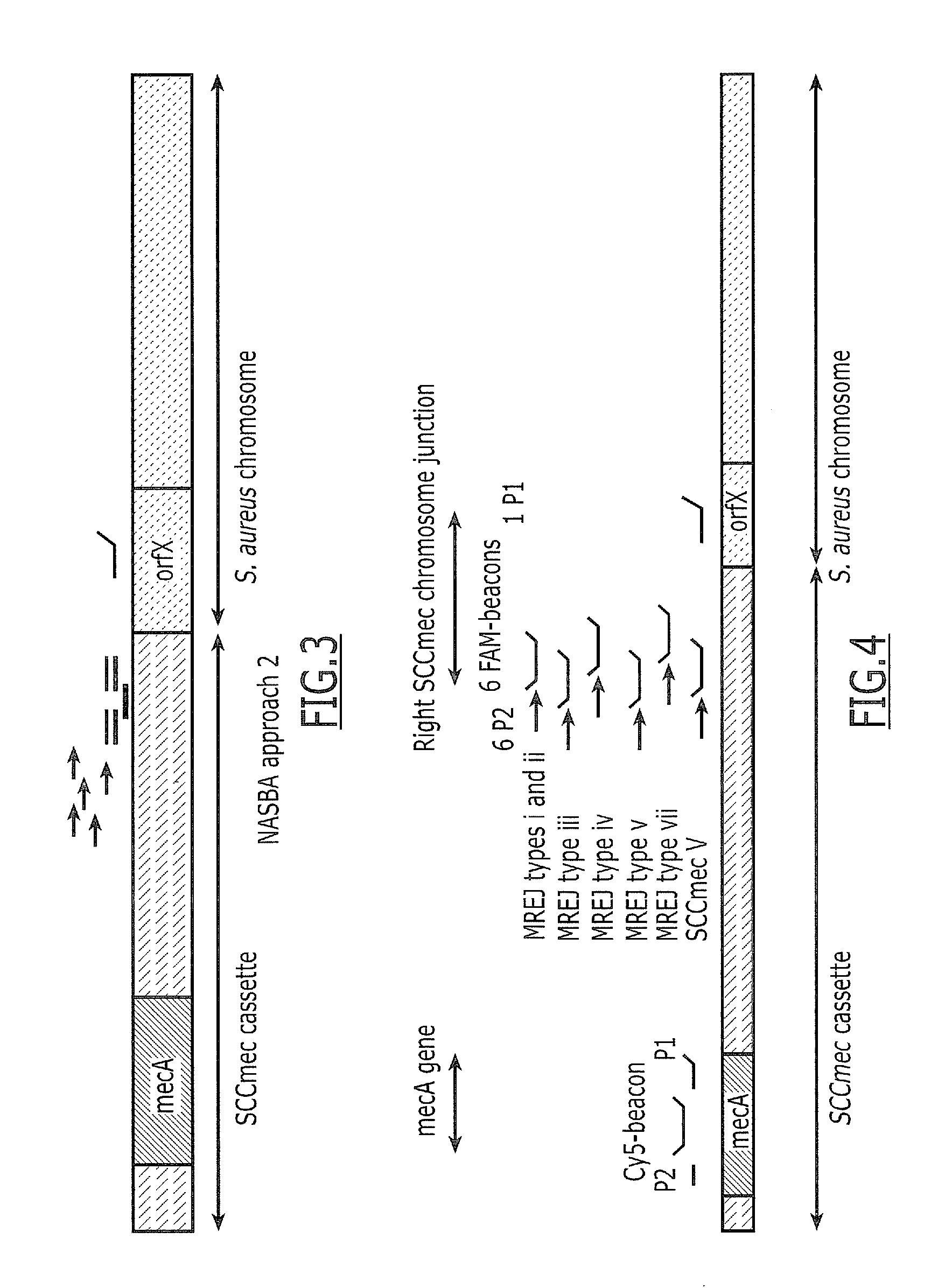
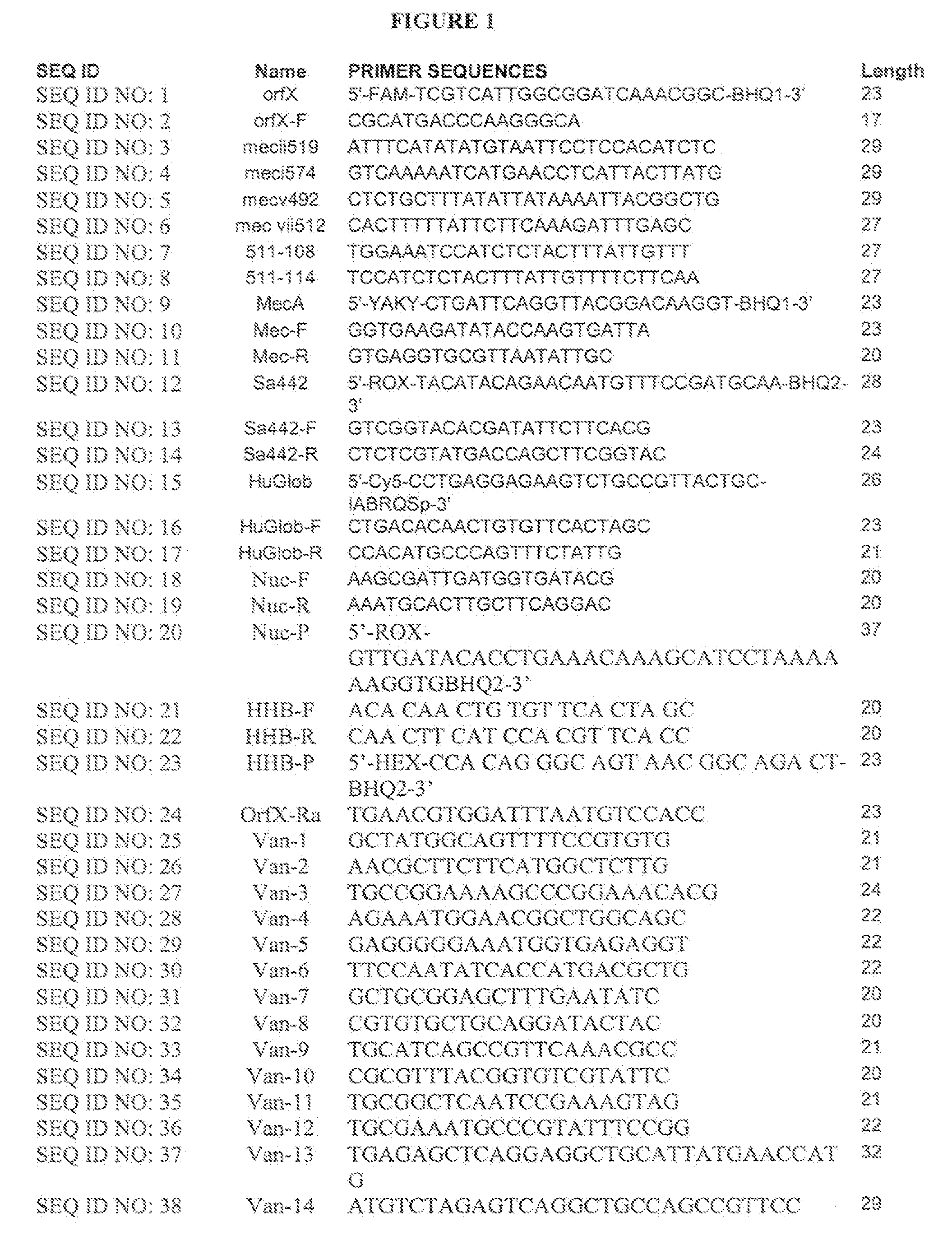
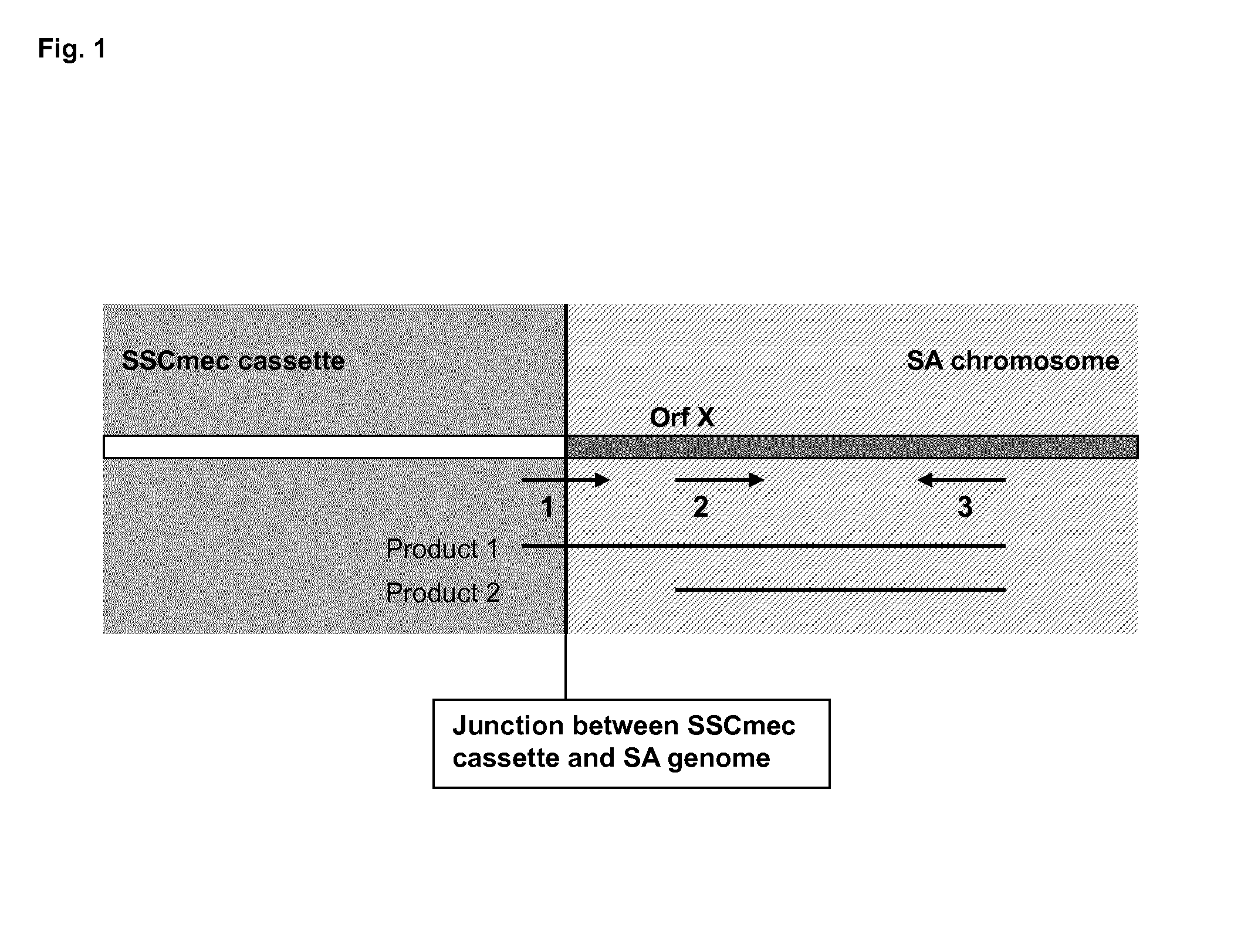
Sequences for detection and identification of methicillin-resistant Staphylococcus aureus (MRSA) of MREJ types xi to xx
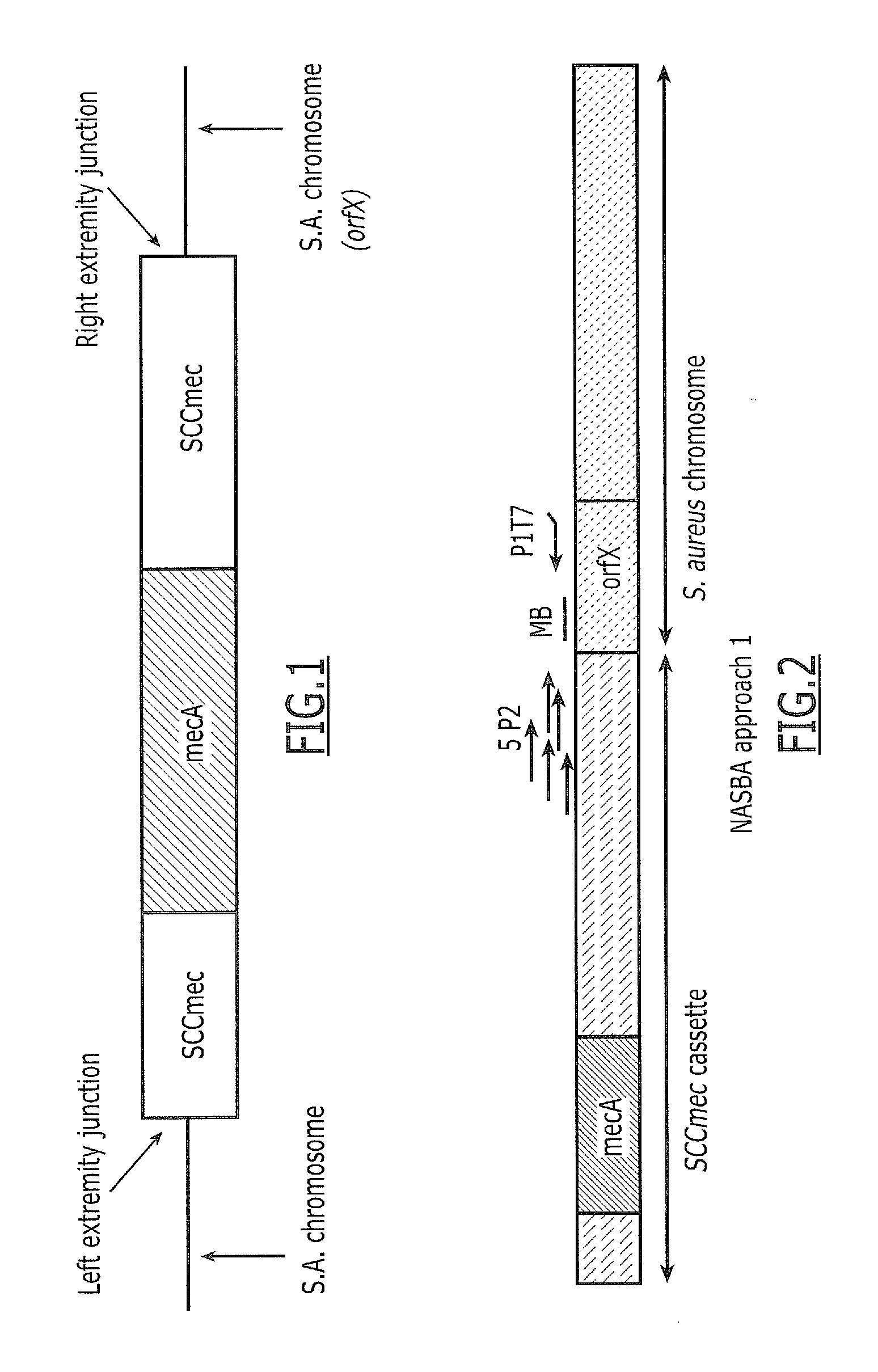
Described herein are novel SCCmec right extremity junction (MREJ) sequences for the detection and / or identification of methicillin-resistant Staphylococcus aureus (MRSA). Disclosed are methods and compositions based on DNA sequences for the specific detection of MREJ sequences designated types xi, xii, xiii, xiv, xv, xvi, xvii, xviii, xix, and xx for diagnostic purposes and / or epidemiological typing.
Owner:GENEOHM SCI INC
New application of patchouli alcohol
ActiveCN103156826AStrong inhibitory activityEffective treatmentAntibacterial agentsCosmetic preparationsBiotechnologyAntibacterial activity
The invention relates to an application of patchouli alcohol in preparation of antibacterial drugs, healthcare food, food, cosmetics, disinfectors or daily chemical articles. The invention also provides the antibacterial drugs, healthcare food, food, cosmetics, disinfectors or daily chemical articles. The patchouli alcohol has good antibacterial activity on pathogenic bacteria or conditional pathogen, can be used for effectively treating bacterium infectious diseases and simultaneously can also effectively antagonize methicillin-resistant staphylococcus epidermidis drug-resistance bacteria; and the pharmacodynamics activity of the patchouli alcohol is even equivalent to that of vancomycin, thus the possibility is provided for slowing down or avoiding the occurrence of the drug-resistance bacteria.
Owner:CHENGDU HUASUN GRP INC LTD +1
Anti-infective agents useful against multidrug-resistant strains of bacteria
The invention relates to novel methods for using macrolide anti-infective agents. The macrolide anti-infective agents demonstrate antibacterial activity against multi-drug resistant strains of bacteria and, in particular, methicillin-resistant Staphylococcus aureus (MRSA). Methods for inhibiting the activity of multi-drug resistant bacterial organisms and methods for treating a bacterial infection caused by such organisms are described herein.
Owner:ABBVIE INC
Detection of antibiotic-resistant microorganisms
ActiveUS20090181395A1Easy to separateReduce morbidityMicrobiological testing/measurementFermentationCoagulase testMethicillin sensitive
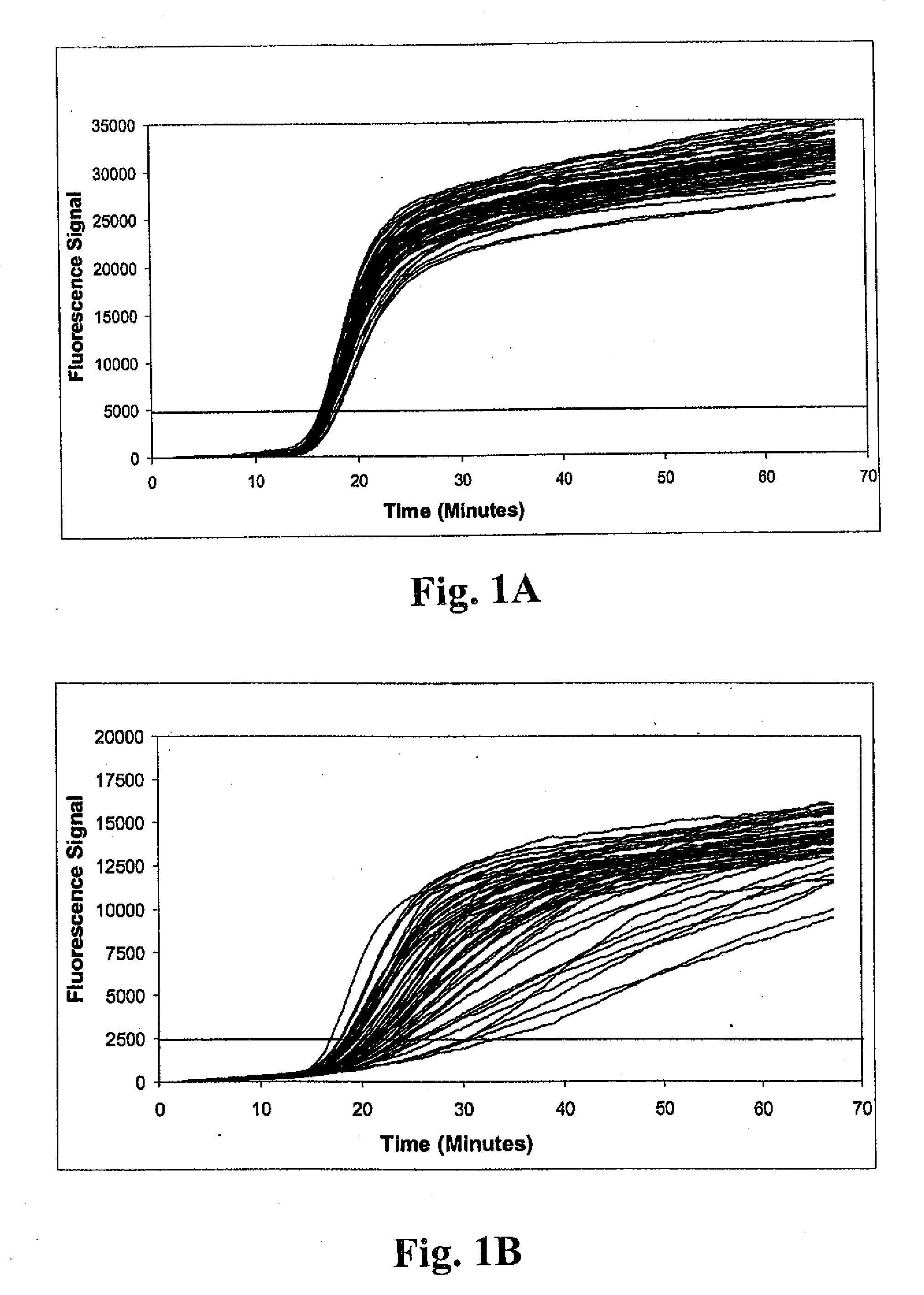
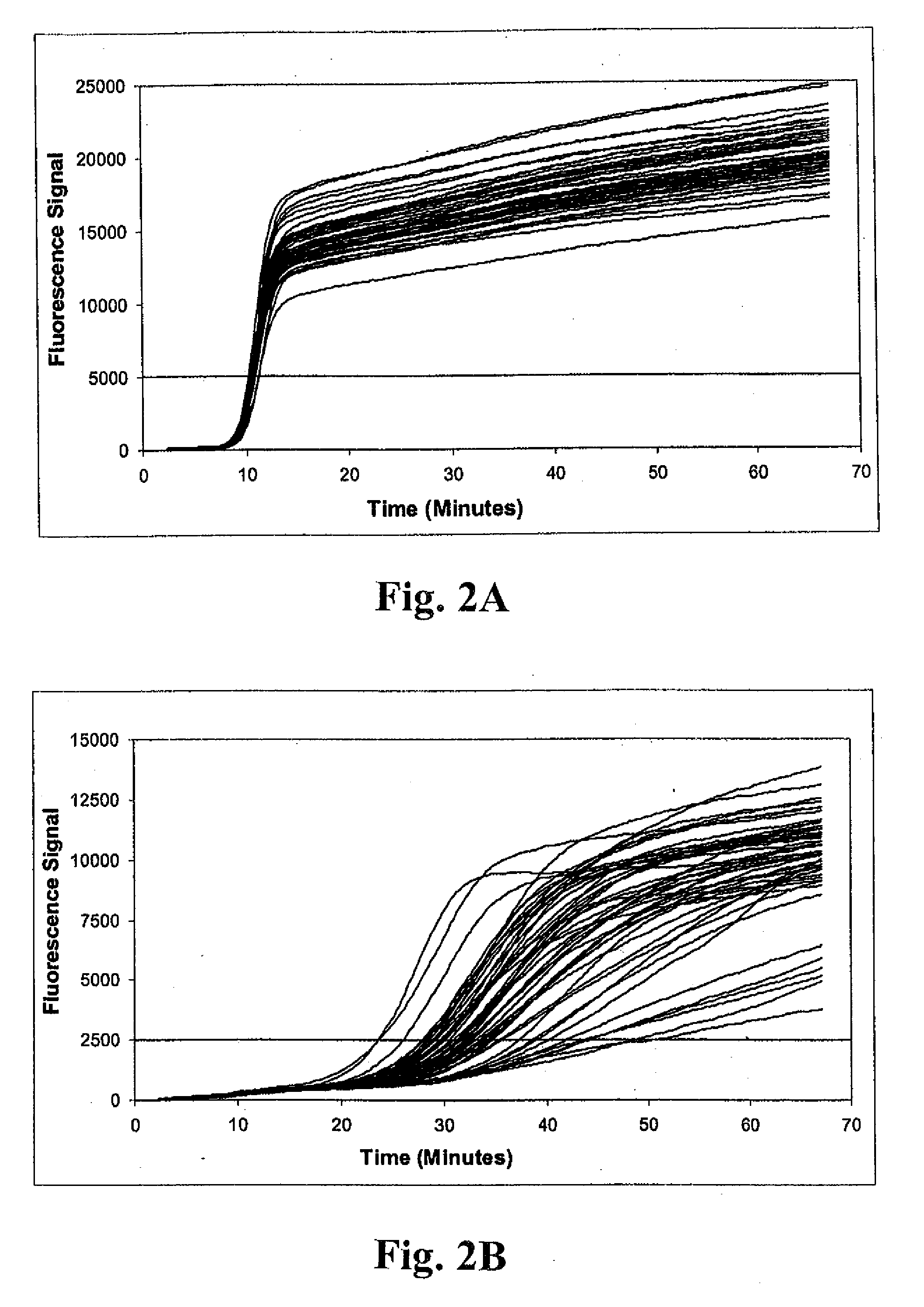
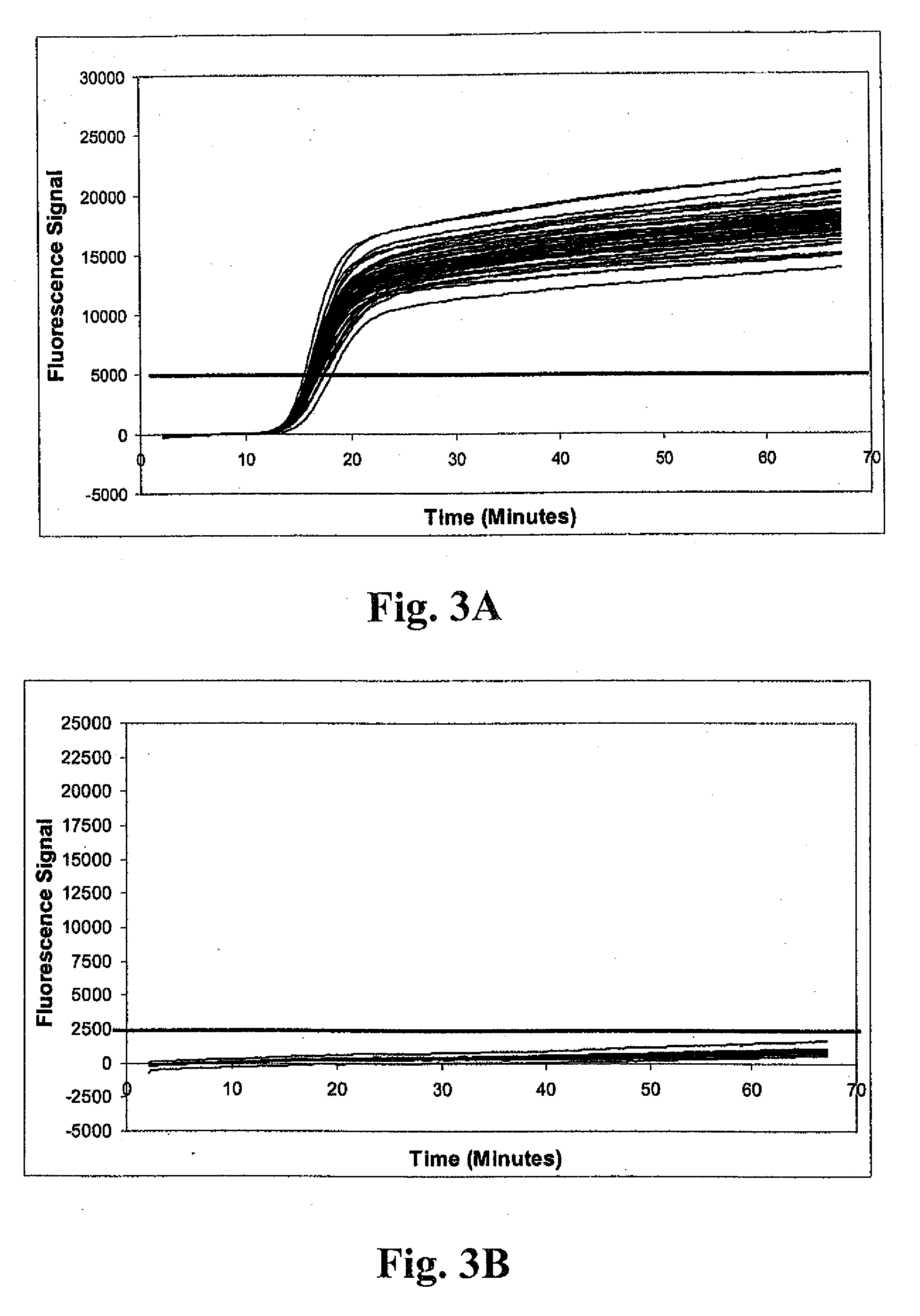
Method of detecting methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) in a nucleic acid coamplification assay. The invention advantageously reduces the incidence of false-positive MRSA determinations in real-time assays by requiring satisfaction of a threshold criterion that excludes certain co-infections from the MRSA determination. The invention further provides for determination of MSSA, even when the MSSA is present in combination with methicillin-resistant coagulase-negative (MR-CoNS) bacteria at high or low levels.
Owner:GEN PROBE INC
Detection of methicillin-resistant Staphylococcus aureus
ActiveUS8367337B2Sugar derivativesMicrobiological testing/measurementMethicillin resistant StaphylococcusStaphyloccocus aureus
The present invention provides improved tests for the detection of methicillin-resistant Staphylococcus aureus. The tests are particularly useful for eliminating false positive results due to the presence of a mixed bacterial population in patient samples.
Owner:BIOMERIEUX SA
2- Arylmethylazetidine Carbapenem Derivatives and Preparation Thereof
InactiveUS20070244089A1High antibacterial activityAntibacterial agentsBiocideAzetidineQuinolone resistance
A 2-arylmethylazetidine carbapenem derivative of formula (I) or a pharmaceutically acceptable salt thereof exhibits a wide spectrum of antibacterial activities against Gram-positive and Gram-negative bacteria and excellent antibacterial activities against resistant bacteria such as methicillinresistant Staphylococcus aureus (MRSA) and quinolone-resistant strains (QRS).
Owner:KOREA RES INST OF CHEM TECH
New application of pogostone or derivatives of pogostone
ActiveCN103416403AOvercoming the pitfalls of drug resistanceEffective againstAntibacterial agentsCosmetic preparationsBiotechnologyChemical products
The invention provides the application of pogostone or derivatives of the pogostone in preparation of antibacterial health food, food, cosmetics, sanitizers or daily chemical products. The invention further provides an antibacterial health food, food, a cosmetic, a sanitizer or a daily chemical product. The pogostone has favorable inhibitory activity to staphylococcus and other pathogenic bacteria or conditioned pathogen, can be used for effective treatment of bacterial infections, and can be effectively against methicillin-resistant drug-resistance bacteria, so that a new choice for clinical medication is provided.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE +1
Methods, compositions and kits for detection and analysis of antibiotic-resistant bacteria
InactiveUS20100255490A1Sugar derivativesMicrobiological testing/measurementResistant bacteriaStaphylococcus
The present invention relates generally to detection of antibiotic-resistant bacteria in a sample. In particular, the invention provides methods, compositions and kits for detecting and analyzing methicillin-resistant Staphylococcus aureus (MRSA) and other methicillin-resistant bacteria in a sample.
Owner:MOLECULAR DETECTION
Antibiotic compositions and methods
InactiveUS20150216874A1Easy to transportImprove survivabilityOrganic active ingredientsBiocideStaphylococcus aureusAntibiotic Y
Disclosed is a novel family of antibiotic that provides bacteria specific targeting, activation and the ability to prevent bacteria mutations that result in bacteria resistance. The compositions and methods of the invention provide for an antibiotic that is effective against Methicillin-resistant Staphylococcus aureus (MRSA).
Owner:TRUOX
Application of clemastine fumarate in preparation for resisting methicillin-resistant staphylococcus aureus (MRSA)
ActiveCN111920805AEnhanced inhibitory effectAchieve inhibitionAntibacterial agentsHeterocyclic compound active ingredientsClemastine FumarateStaphylococcus aureus
The invention particularly relates to an application of clemastine fumarate in an anti-MRSA preparation. The MRSA has high toxicity, shows drug resistance to most clinically common antibiotic drugs, is widely distributed in a daily environment, and is a troublesome pathogenic strain. In order to develop an active substance with an inhibition effect on the drug-resistant bacteria, the invention screens and verifies that the clemastine fumarate has a good inhibition effect on MRSA from clinical drugs, and is expected to be applied to the development of anti-MRSA infection related drugs.
Owner:MATERNAL & CHILD HEALTH CARE HOSPITAL OF SHANDONG PROVINCE SHANDONG UNIV
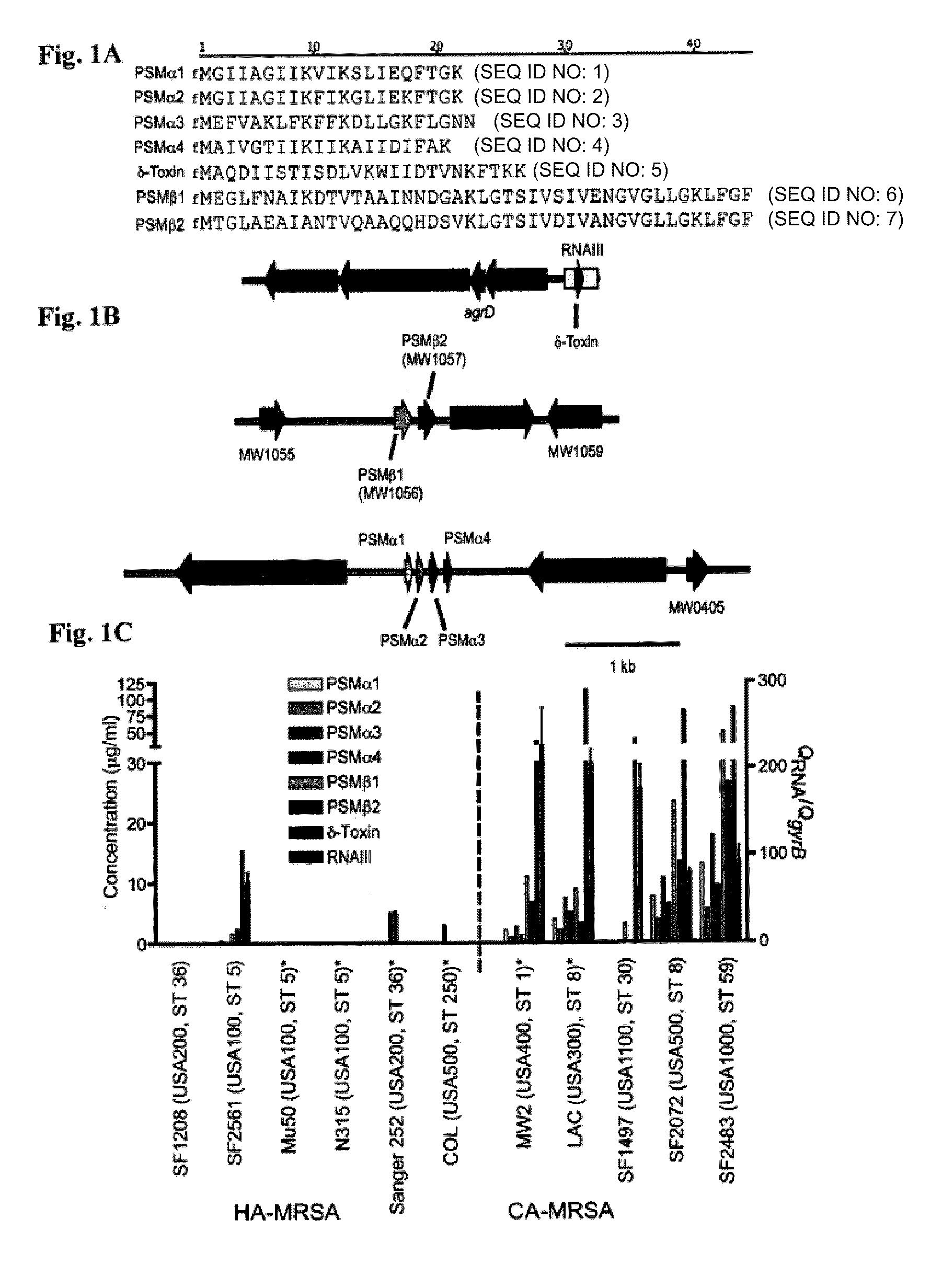
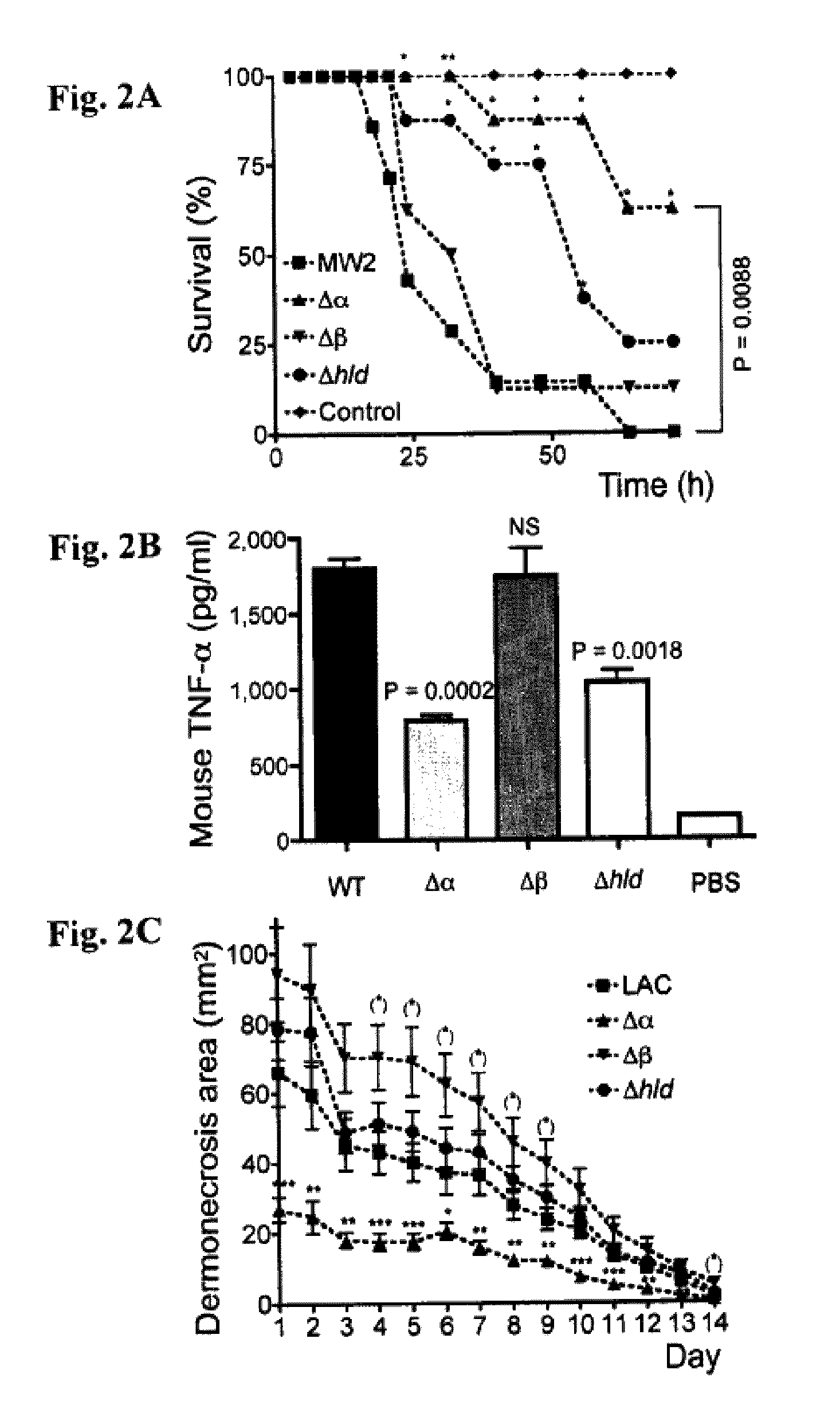
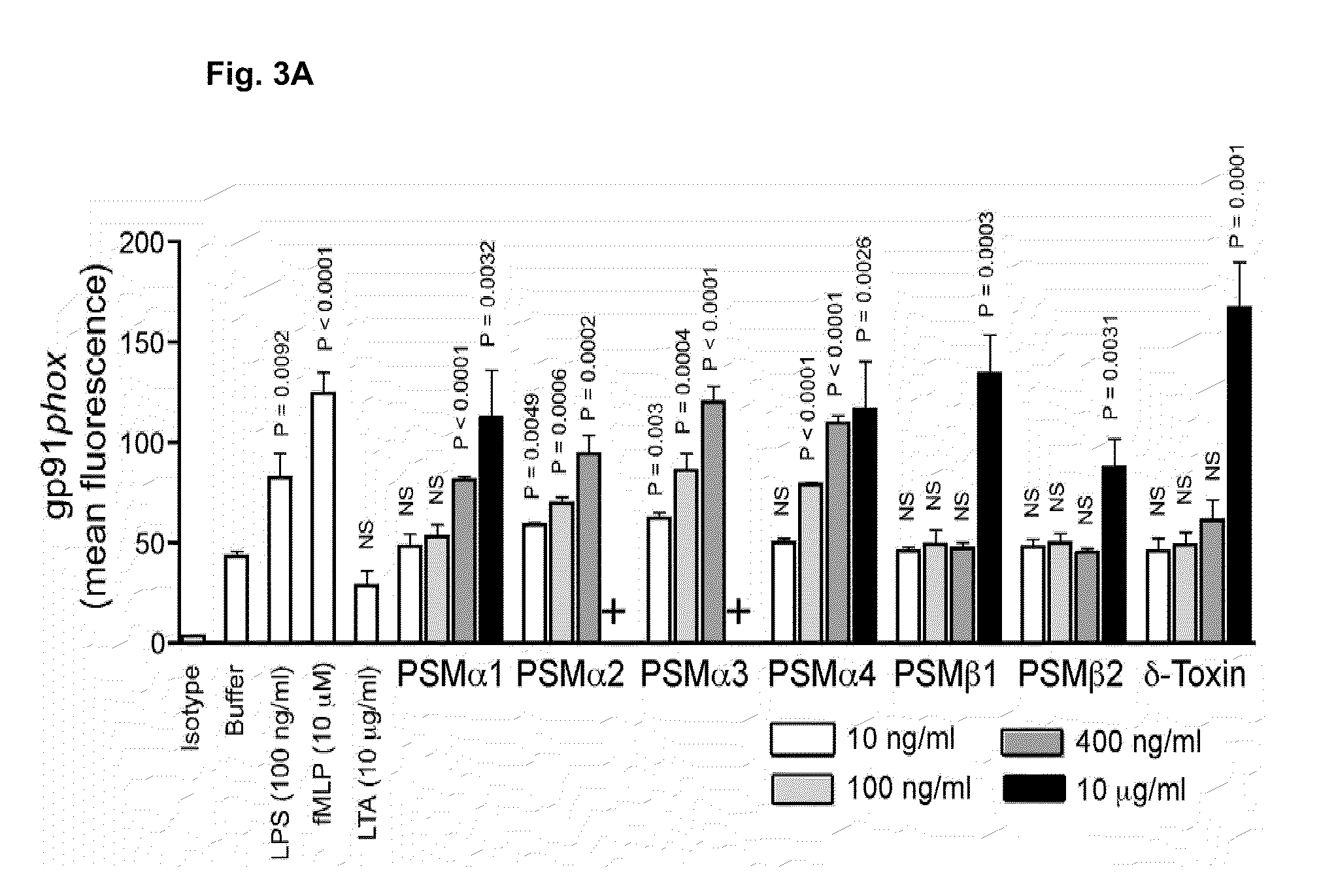
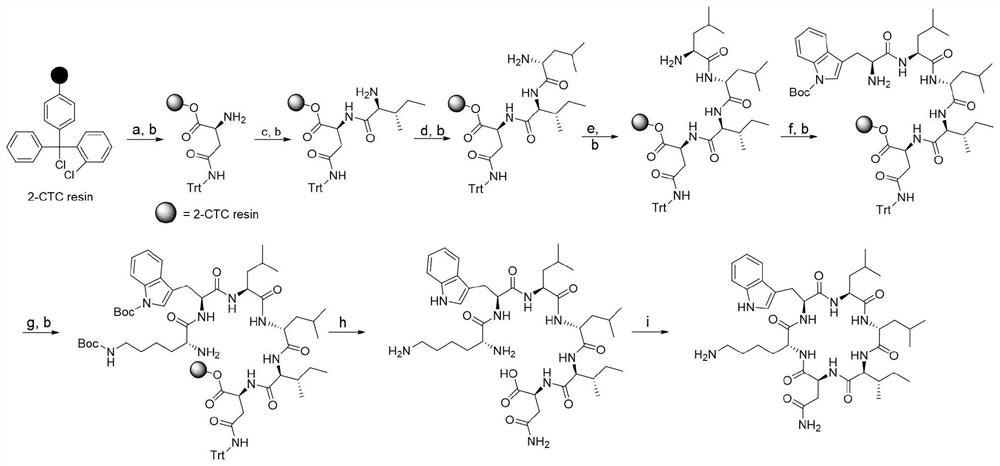
PSM peptides as vaccine targets against methicillin-resistant Staphylococcus
This disclosure concerns compositions and methods for the treatment and inhibition of infectious disease, particularly methicillin-resistant Staphylococcus. In certain embodiments, the disclosure concerns immunogenic peptides, for instance PSM peptides, which can be used to induce protective immunity against methicillin-resistant Staphylococcus. Also disclosed are methods of detecting methicillin-resistant staphylococcus in a sample, and methods of diagnosing methicillin-resistant staphylococcus in a subject.
Owner:UNITED STATES OF AMERICA
Cyclic hexadepsipeptide compound desotamide A4 and application thereof in preparation of antibacterial drugs
ActiveCN113150077AStrong inhibitory activityHigh activityAntibacterial agentsPeptidesCyclic peptideStaphylococcus haemolyticus
The invention discloses a cyclic hexapeptide compound desotamide A4 and an application thereof in preparation of antibacterial drugs. The structural formula of the compound desotamide A4 is as shown in formula (I) . The cyclic hexapeptide desotamide A4 disclosed by the invention has a broad-spectrum anti-gram pathogenic bacterium effect. The test result shows that the desotamide A4 has good inhibitory activity (MIC is 8-32 [mu] g / mL) on a plurality of clinical drug-resistant staphylococcus aureus, enterococcus faecalis, micrococcus luteus, bacillus subtilis, simulated staphylococcus and staphylococcus haemolyticus including methicillin-resistant staphylococcus aureus; compared with an unmodified parent natural cyclic peptide compound desotamide A, the activity of the cyclic hexapeptide desotamide A4 disclosed by the invention is enhanced by 2-4 times, and the cyclic hexapeptide desotamide A4 has an important value in research and development of antibacterial drugs.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Streptomyces-derived antimicrobial compound and method of using same against antibiotic-resistant bacteria
InactiveUS20100227918A1Useful in treatmentEffective treatmentAntibacterial agentsBiocideMethicillin resistanceFermentation
The present invention relates to a novel antimicrobial compound of lactoquinomycin that is highly effective against many antibiotic-resistant gram-positive bacteria; namely, methicillin-resistant and vancomycin-resistance Staphylococcus aureus, vancomycin-resistant Enterococcus faecilis and Mycobacteria. The present invention also relates to a fermentation process of culturing a Streptomyces strain to prepare the antimicrobial compound and its use in killing the antibiotic-resistant bacteria.
Owner:TARO PHARMA INDS
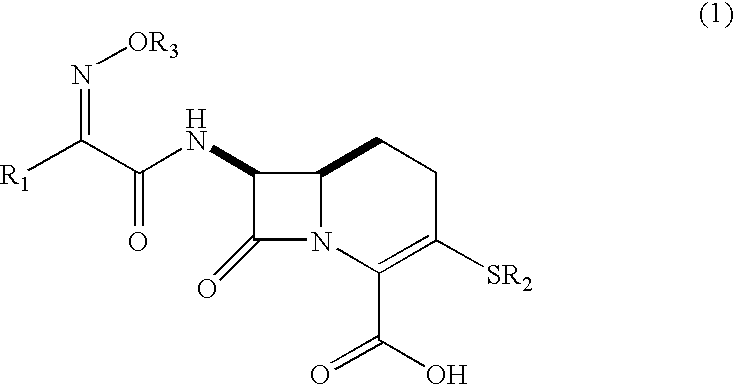
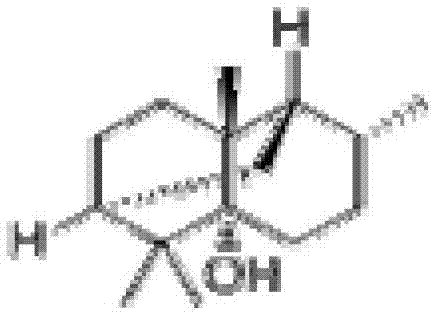
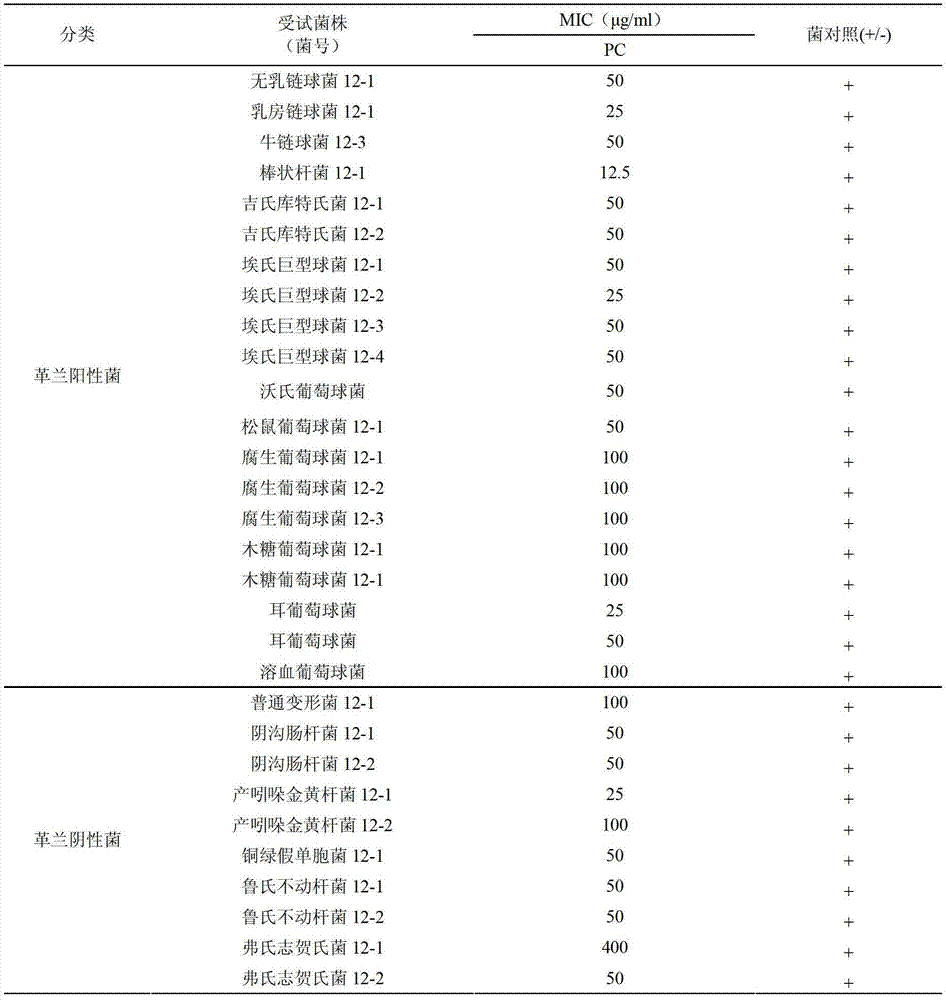
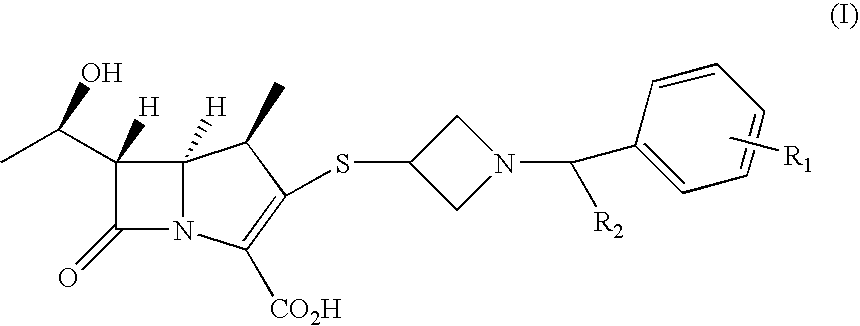
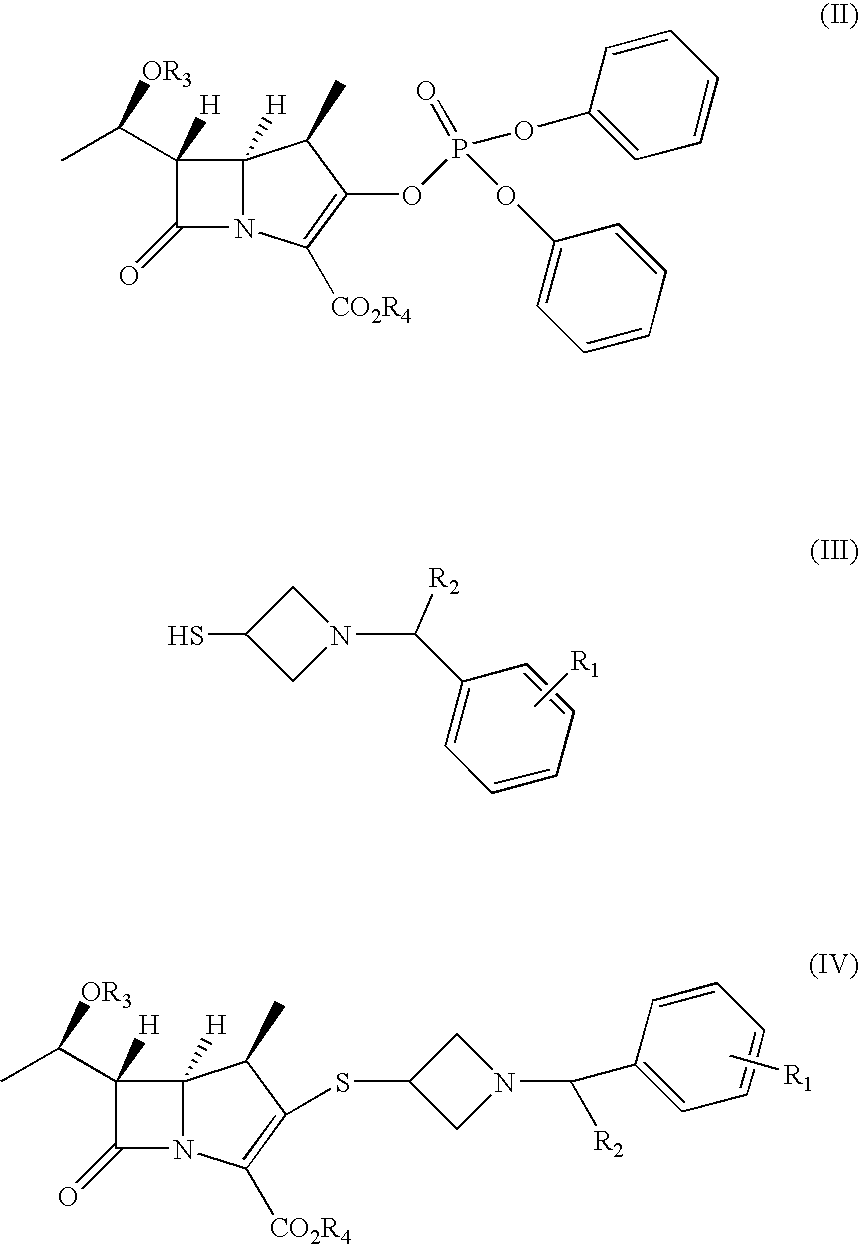
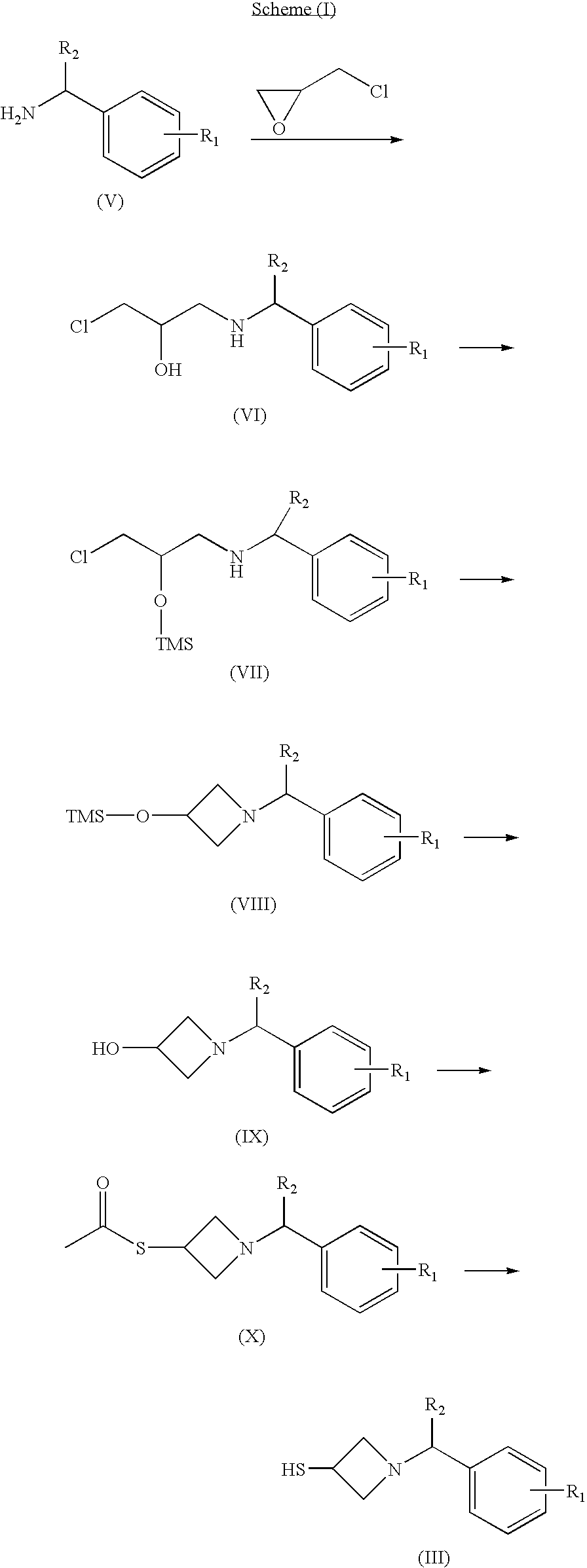
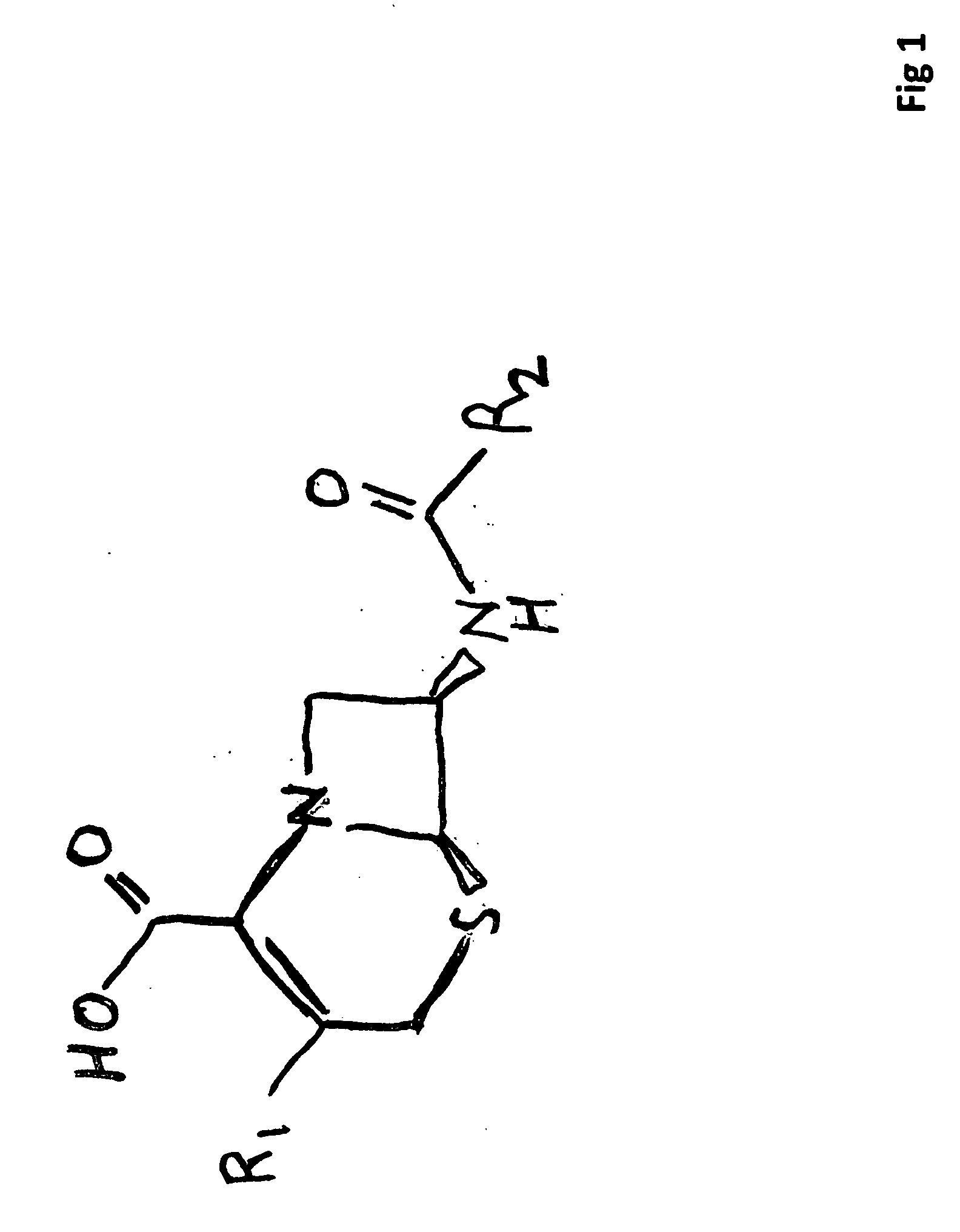
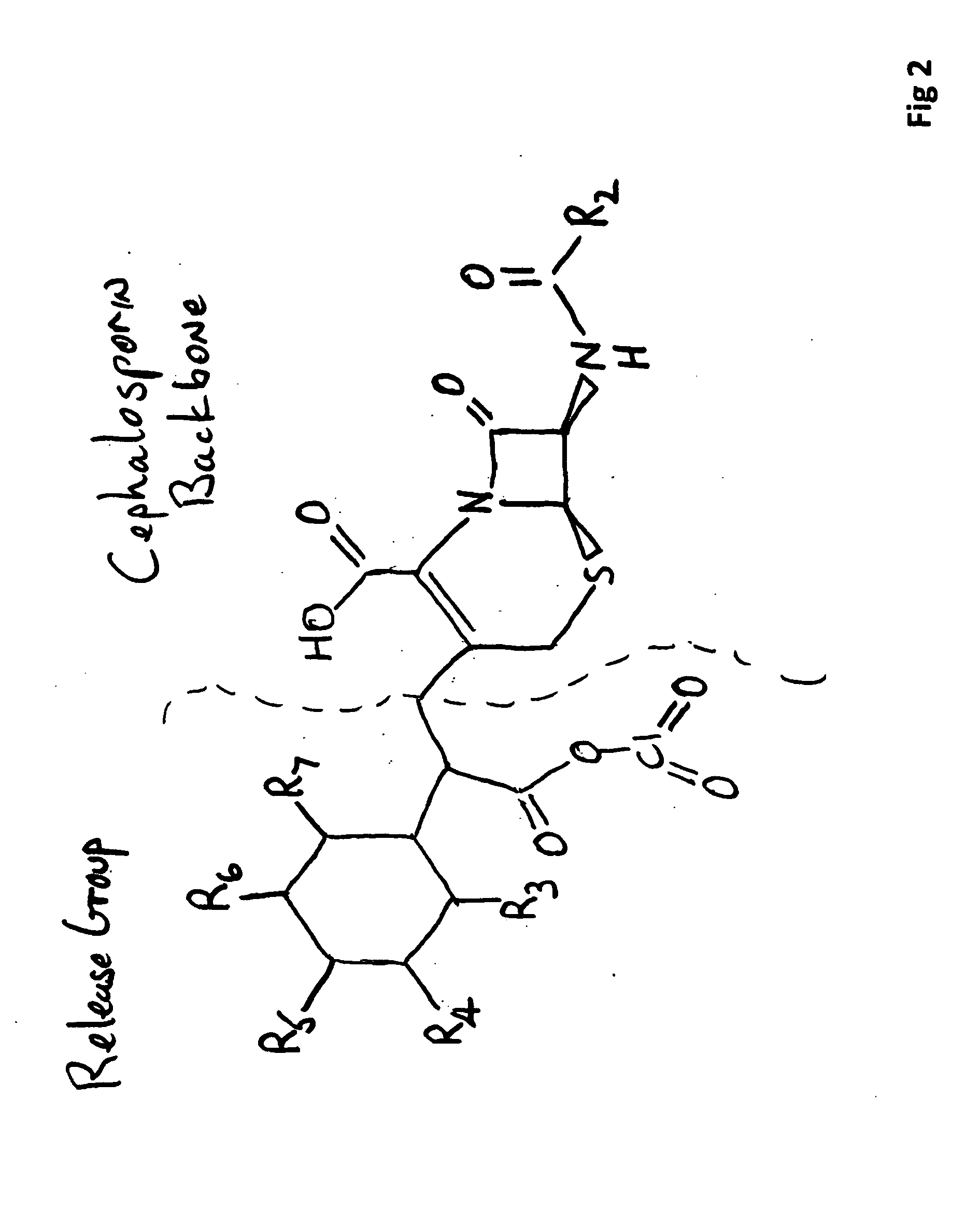
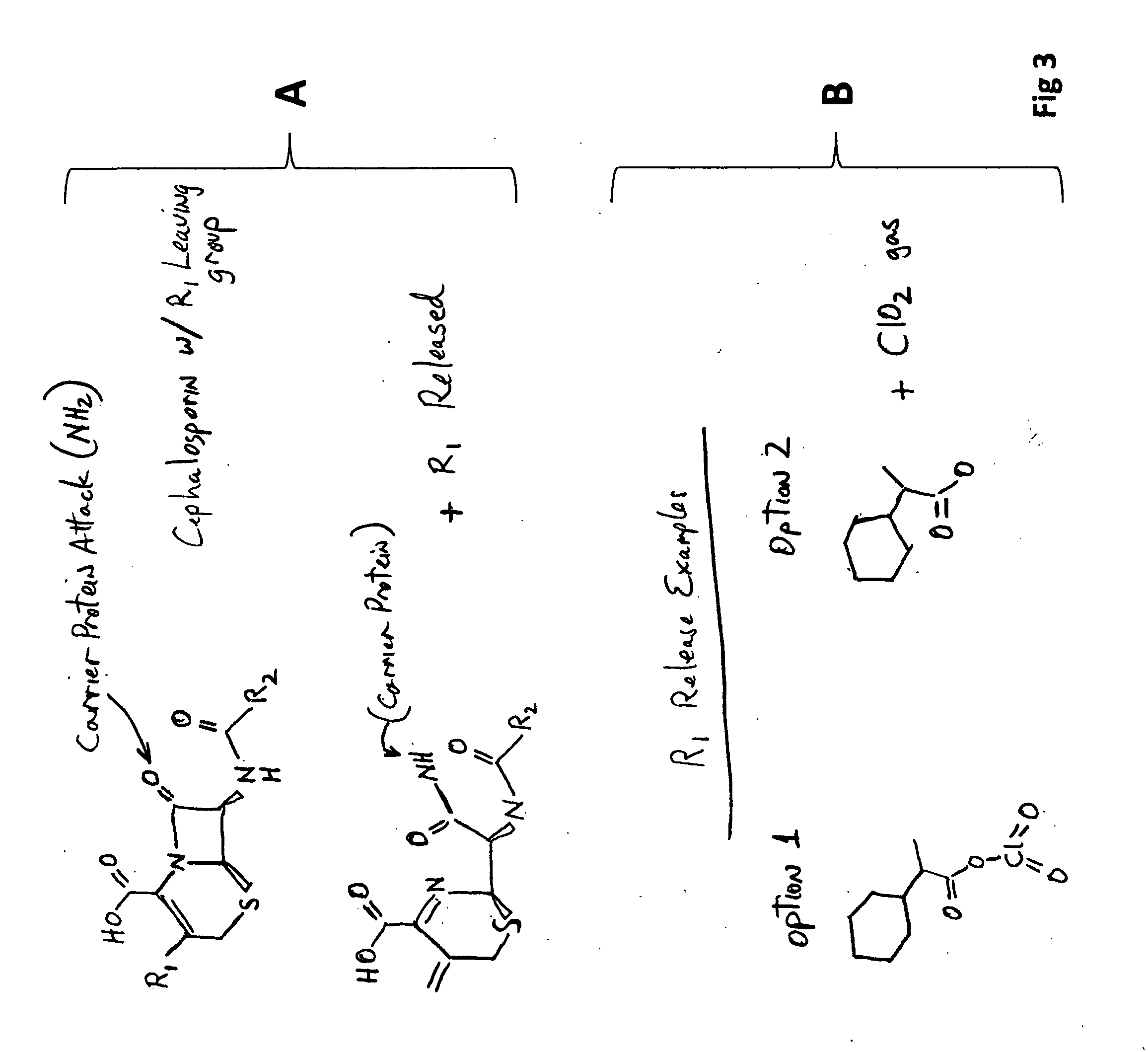
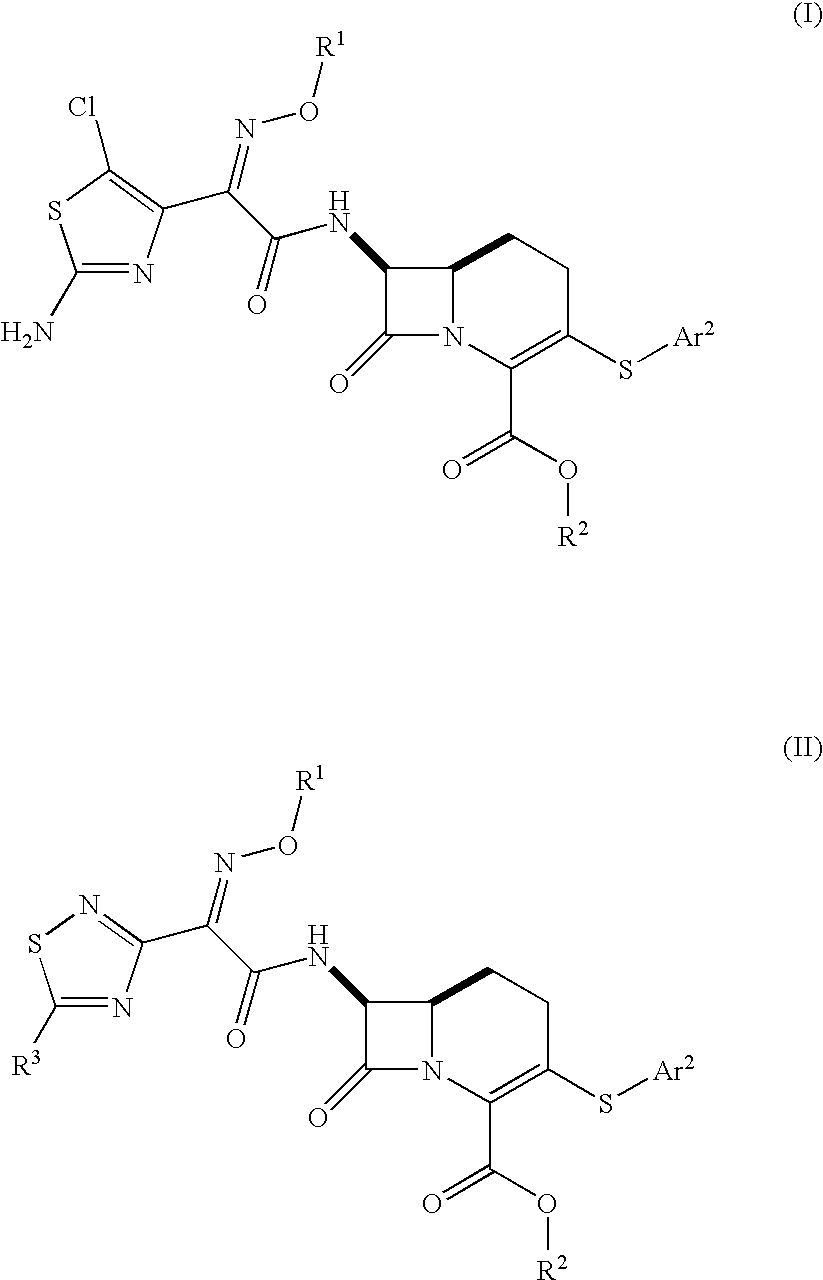
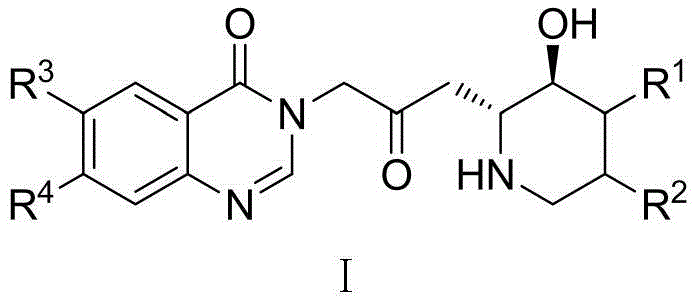
Carbacephem β-lactam antibiotics
Carbacephem β-lactam antibiotics having the following chemical structures (I) and (II) are disclosed:including stereoisomers, pharmaceutically acceptable salts, esters and prodrugs thereof, wherein Ar2, R1, R2 and R3 are as defined herein. The compounds are useful for the treatment of bacterial infections, in particular those caused by methicillin-resistant Staphylococcus spp.
Owner:ACHAOGEN
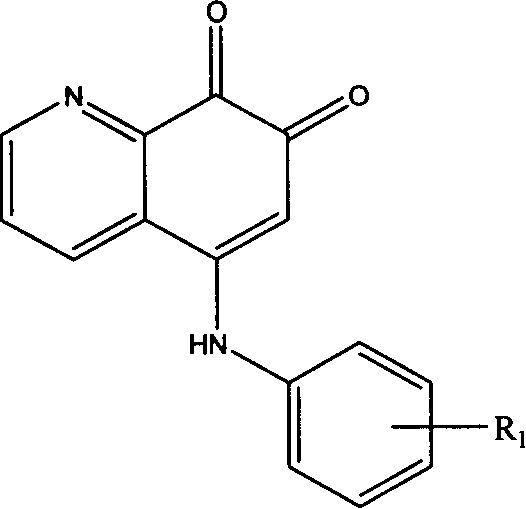
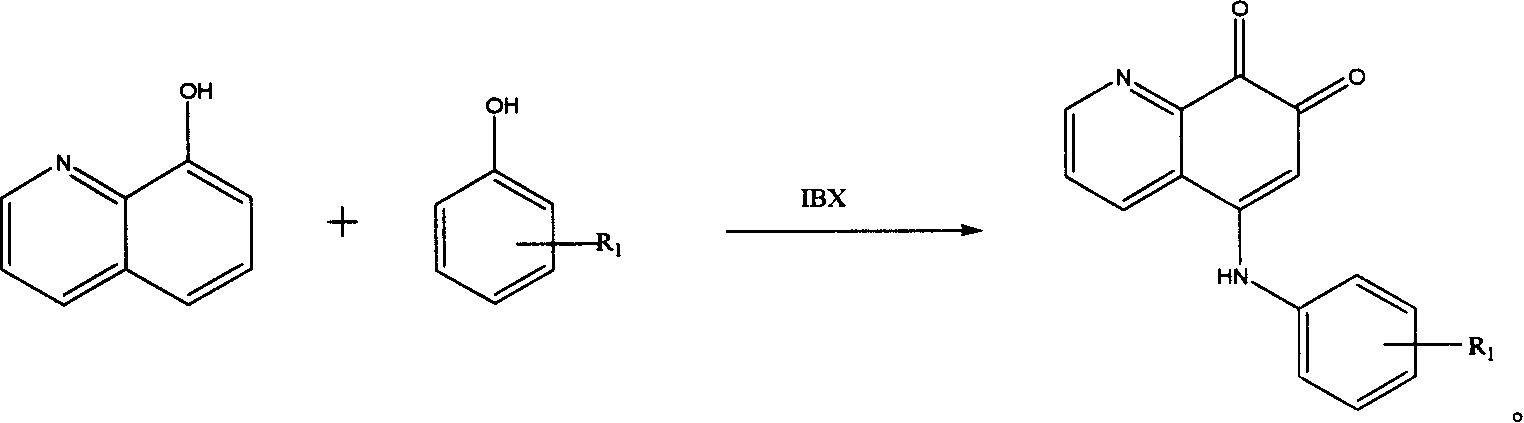
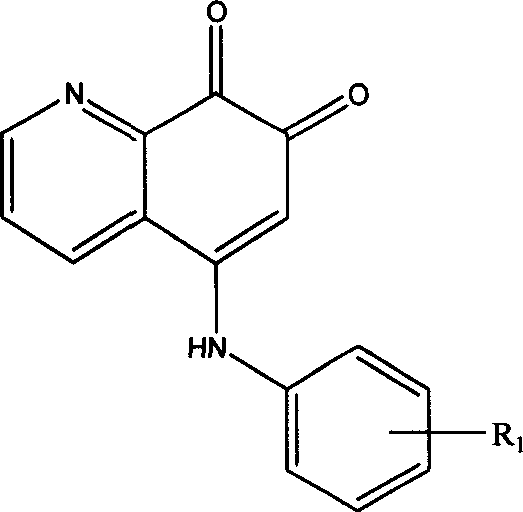
5-arylamino quinolyl-7,8-dione derivative and its application in preparing antibiotic medicine
InactiveCN1974557AEnhanced inhibitory effectReduce inhibitionAntibacterial agentsOrganic chemistryChemical structureAntimicrobial drug
The present invention discloses 5-arylamino quinolyl-7, 8-dione derivative with the chemical structure expression as shown. The present invention also relates to the synthesis and application in preparing antibiotic medicine, especially medicine for antagonizing methicillin resisting Staphylococcus aureus (MRSA), of the derivative. Experiment shows that the 5-arylamino quinolyl-7, 8-dione derivative has obvious bacteriostasis effect on some Gram positive bacteria, especially on methicillin resisting Staphylococcus aureus (MRSA), and may be used in preparing effective antibiotic medicine.
Owner:GUANGDONG UNIV OF TECH
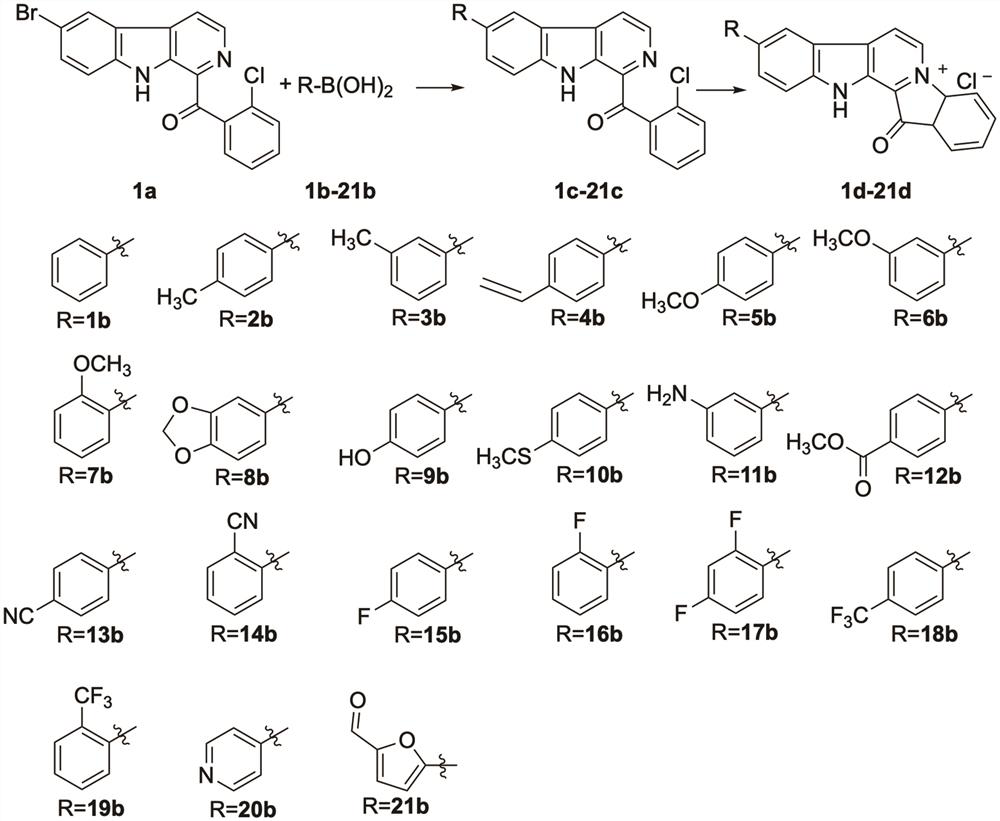
Fascaplysin derivative, preparation method and application of Fascaplysin derivative in MRSA (Methicillin Resistant Staphylococcus Aureus) resistance
PendingCN114262330AQuick buildRapid construction with structural diversityAntibacterial agentsOrganic chemistryAntimicrobial drugBoronic acid
The invention discloses a method for rapidly preparing a Fascaplysin derivative through a Suzuki-Miyaura coupling reaction and an application of the Fascaplysin derivative in resisting methicillin-resistant staphylococcus aureus (MRSA ATCC43300), and particularly discloses a method for rapidly preparing the Fascaplysin derivative through the Suzuki-Miyaura coupling reaction. The preparation method comprises the following steps: (1) carrying out regioselective Suzuki-Miyaura coupling on phenylboronic acid or heterocyclic boronic acid with different substituent groups and a dihalo-substituted raw material substrate, so as to obtain a series of Fascaplysin derivative precursor compound beta-carboline; and (2) carrying out intramolecular ring closing reaction on beta-carboline through high-temperature reaction, so as to obtain a series of Fascaplysin derivatives. According to the preparation method disclosed by the invention, a Fascaplysin derivative compound library with structural diversity can be quickly and efficiently constructed, and compared with the traditional Fascaplysin derivative synthesis, the preparation time is greatly shortened, and the synthesis efficiency is improved. The synthesized Fascaplysin derivative shows strong antibacterial activity, inhibits the formation of a biological membrane, and is an antibacterial drug candidate compound with a good application prospect.
Owner:NINGBO UNIV
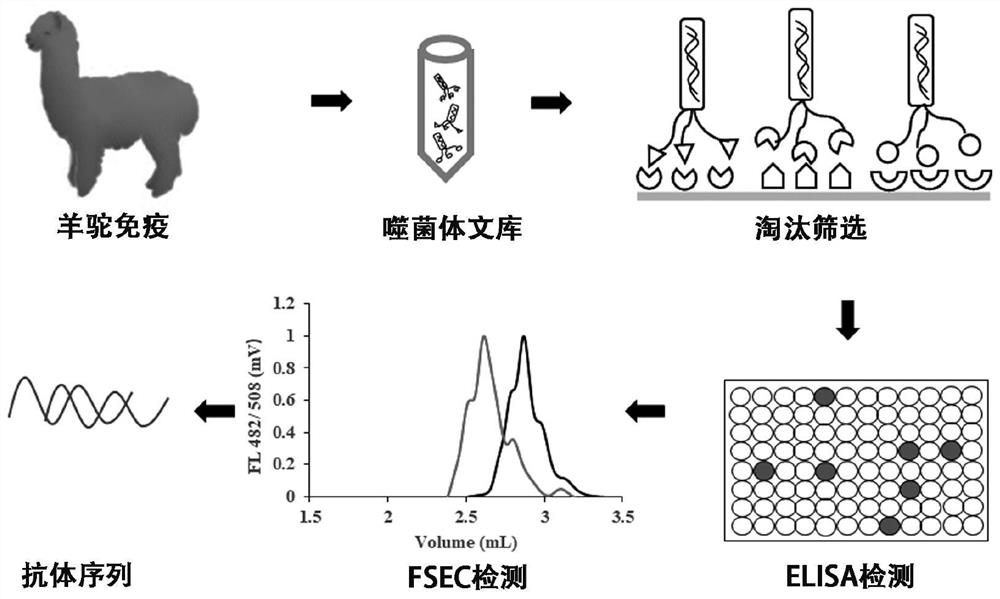
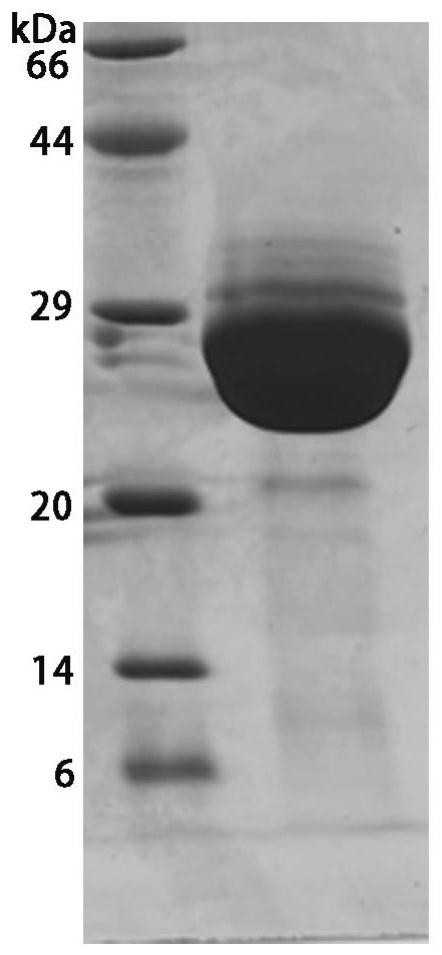
Nano antibody for resisting methicillin-resistant staphylococcus as well as preparation method and application of nano antibody
InactiveCN113980127AImprove specific recognition abilityImprove specific binding abilityAntibacterial agentsBiological material analysisComplementarity determining regionBiomedicine
The invention provides a nano antibody targeting a methicillin-resistant staphylococcus LspA protein as well as a preparation method and application of the nano antibody. The provided nano antibody mainly recognizes a binding motif region combined with the methicillin-resistant staphylococcus LspA, and comprises a framework region FR and complementary determining regions CDR1, CDR2 and CDR3. One specific amino acid sequence of the nano antibody is SEQ ID NO: 1. The nano antibody disclosed by the invention can be efficiently and specifically combined with methicillin-resistant staphylococcus LspA protein, and the affinity can reach a nanomole level; and meanwhile, the nano antibody provided by the invention can obtain an excellent effect in methicillin-resistant staphylococcus detection or diseases caused by methicillin-resistant staphylococcus, and can be applied to the fields of biology and medicine.
Owner:南京中爱人工智能与生命科学研究院有限公司
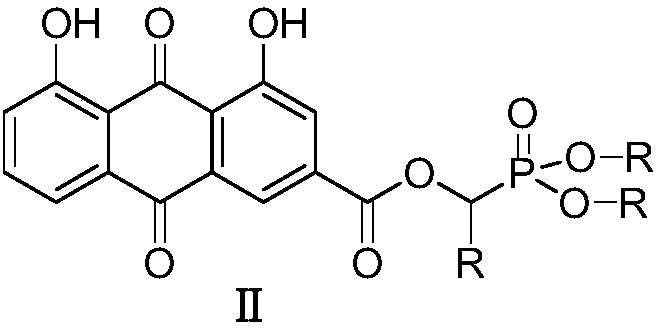
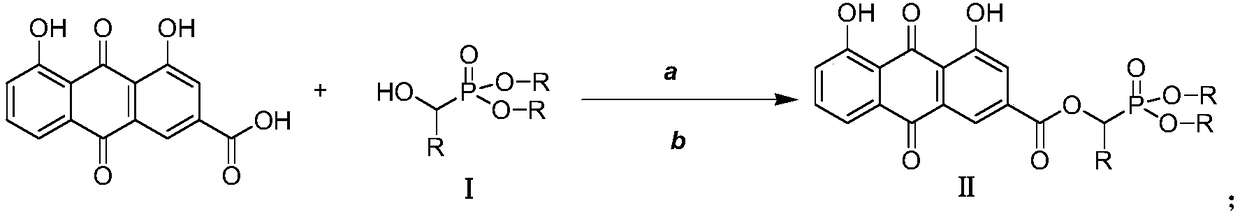
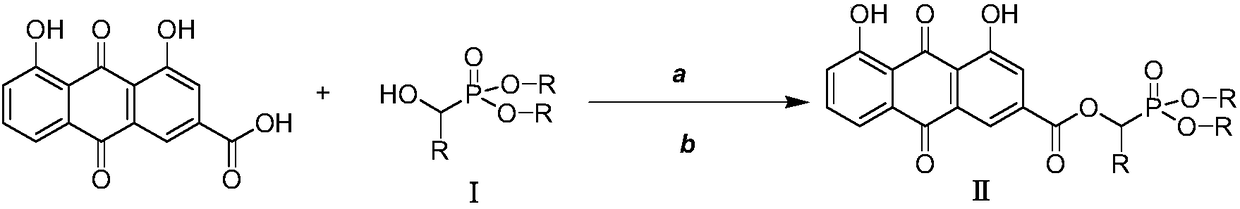
Rhubarb acid ester derivative and preparation method and application thereof
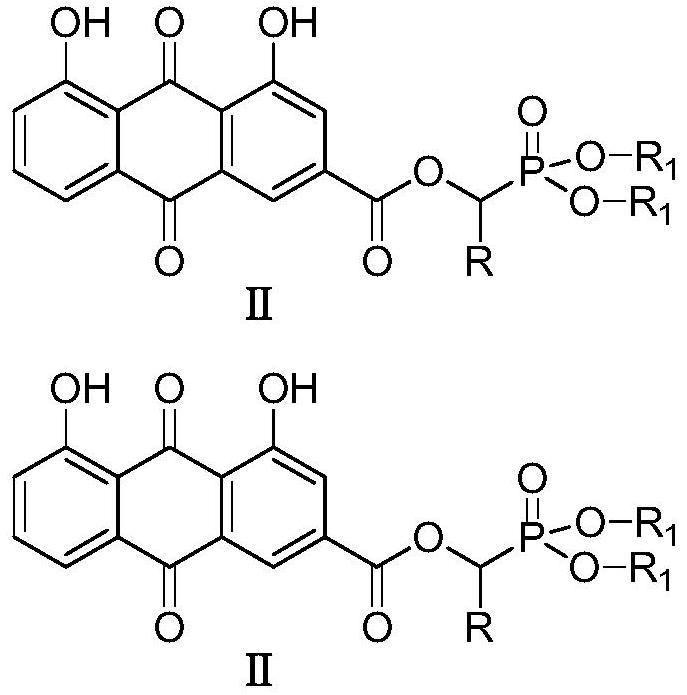
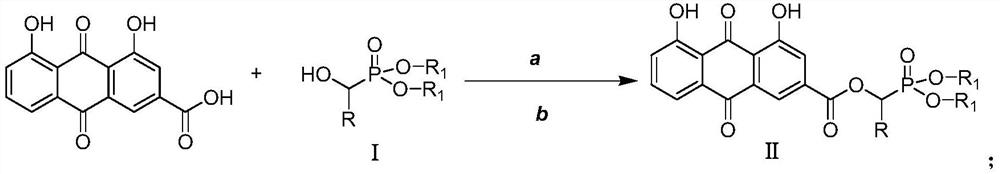
ActiveCN109053798AImprove antibacterial propertiesNovel structureAntibacterial agentsOrganic active ingredientsAntimicrobial actionAntibacterial activity
The invention discloses a rhubarb acid ester derivative in the field of antibiotic compounds. The chemical structural formula of rhubarb acid ester is as shown in the formula (II), the formula (II) isshown in the description, wherein R is a chain substituent group of 1-5 carbon atoms or a naphthenic base or an aromatic hydrocarbon group of 3-6 carbon atoms. An antibiotic activity test proves thatthe rhubarb acid ester derivative with the novel structure has the antibiotic activity on methicillin-resistant staphylococcus aureus (MRSA). A plurality of targets have the outstanding antibiotic effect on methicillin-resistant staphylococcus aureus (MRSA), are superior to the reference drug oxacillin and is close to the reference drug vancomycin, and the rhubarb acid ester derivative can be served as a candidate compound to resist methicillin-resistant staphylococcus aureus (MRSA) and be researched.
Owner:ZUNYI MEDICAL UNIVERSITY
Beta-lactam compounds and process for preparing the same
InactiveUS6265396B1High antibacterial activityAntibacterial agentsBiocideAntibacterial activityBeta-lactam
A beta-lactam compound of the formula:wherein R1 is lower alkyl or OH-substituted lower alkyl, R2 is H or lower alkyl, X is O, S or NH, n is 1 to 3, R3 is -C(Ra)=NH (Ra is H, lower alkyl or substituted lower alkyl), or a salt thereof, or an ester thereof. These compounds show excellent antibacterial activity against Gram-positive bacteria, particularly against methicillin-resistant Staphylococci and methicillin-resistant and coagulase-negative Staphylococci.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
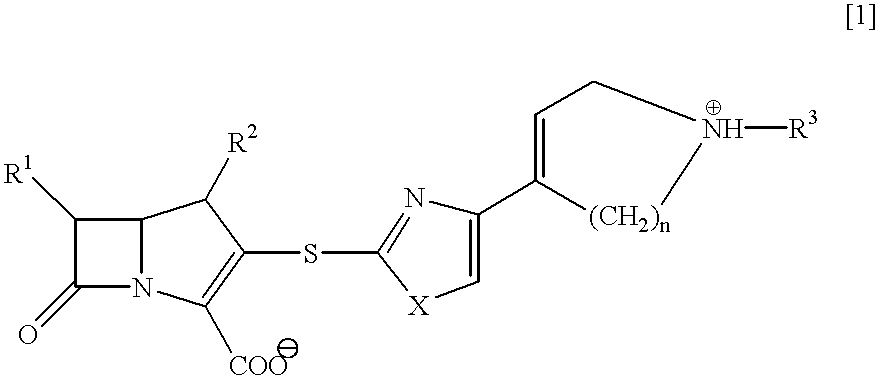
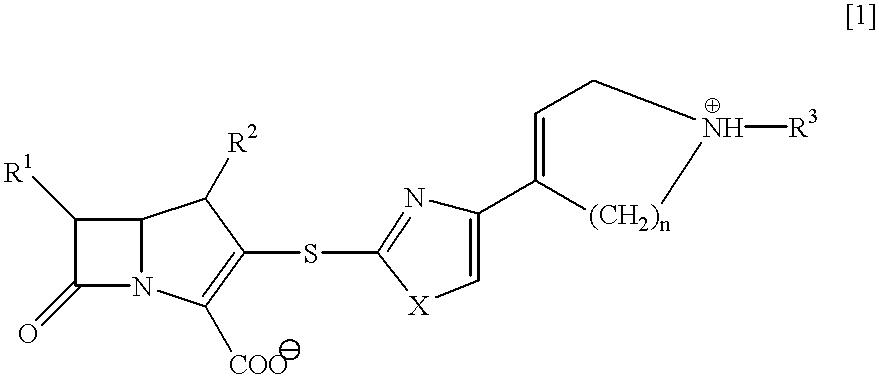
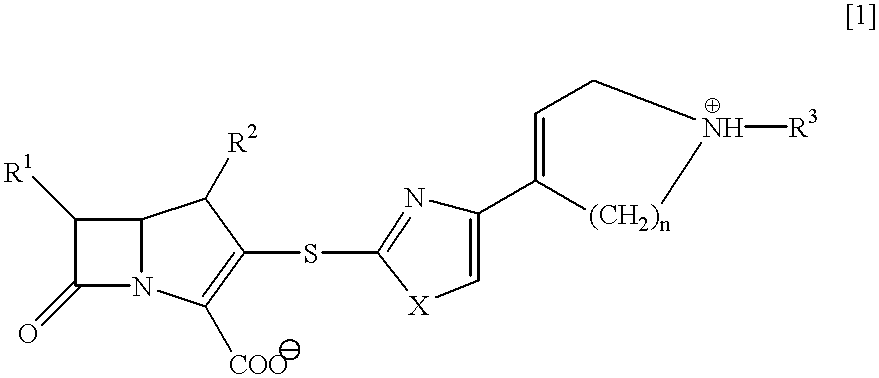
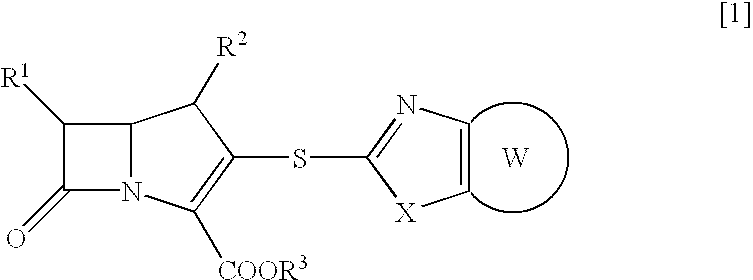
Beta-lactam compounds and process for producing the same
InactiveUS6599895B1High antibacterial activityUseful for medicamentAntibacterial agentsBiocideNitrogenGram-positive bacterium
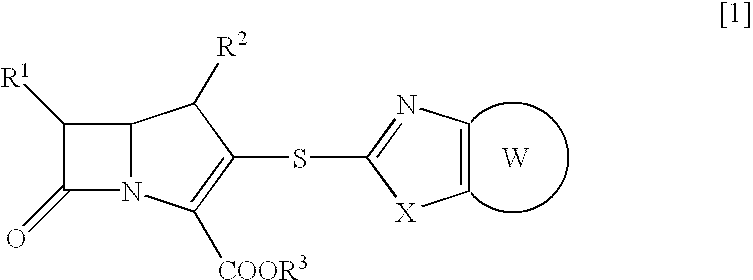
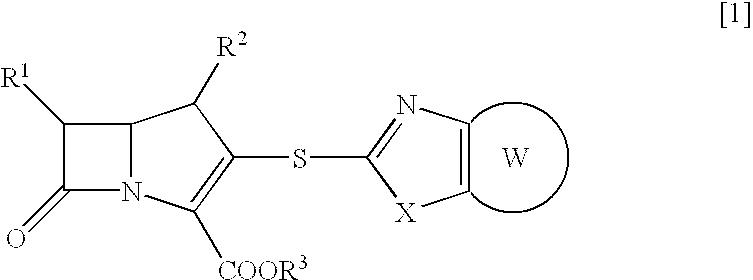
Novel beta-lactam compound of the formula [1]:wherein R1 is lower alkyl or hydroxy-substituted lower alkyl, R2 is H or lower alkyl, X is O or, S, R3 is H, metal or protecting group, W is a 6- or 7-membered nitrogen-containing heterocycle optionally being substituted at carbon atoms. Said beta-lactam compound shows excellent antibacterial activity against Gram-positive bacteria, particularly against methicillin-resistant Staphylococcus aureus and methicillin-resistant and coagulase-negative Staphylococcus aureus.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
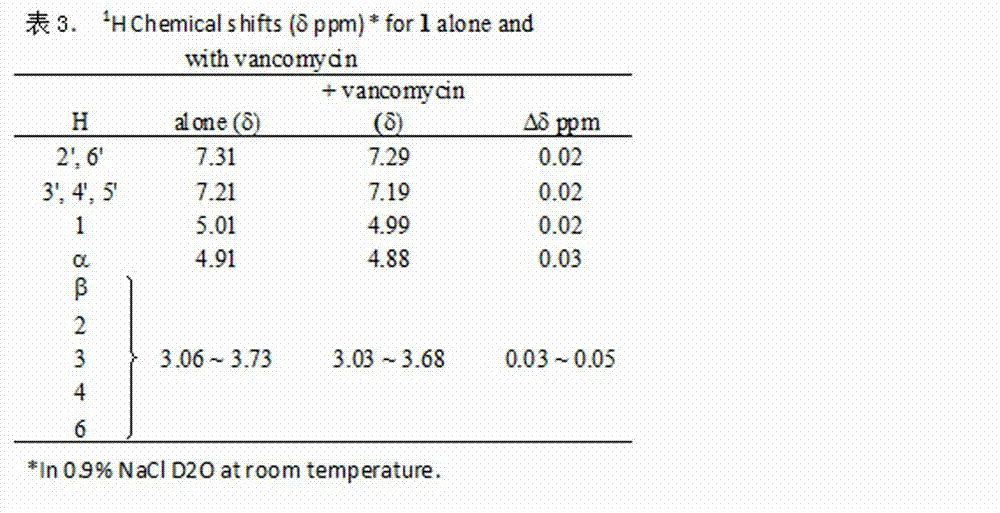
Component for forming bacterium-resisting and efficacy-enhancing inclusion compound together with vancomycin and preparation method and application thereof
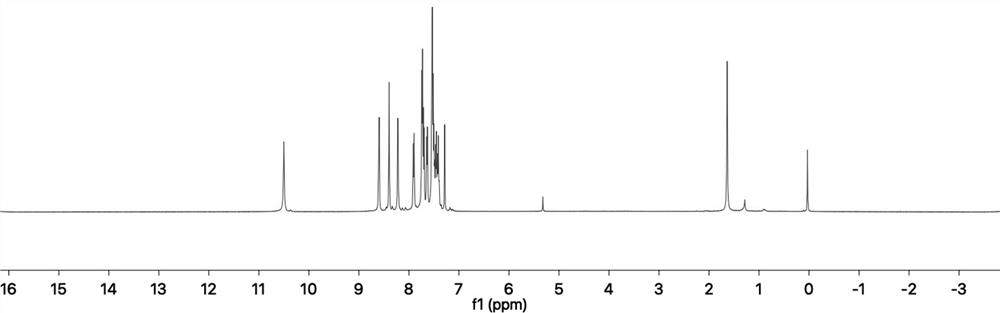
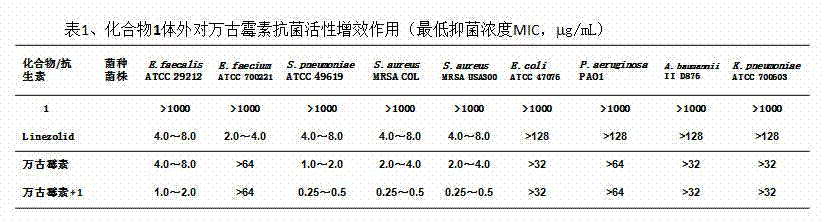
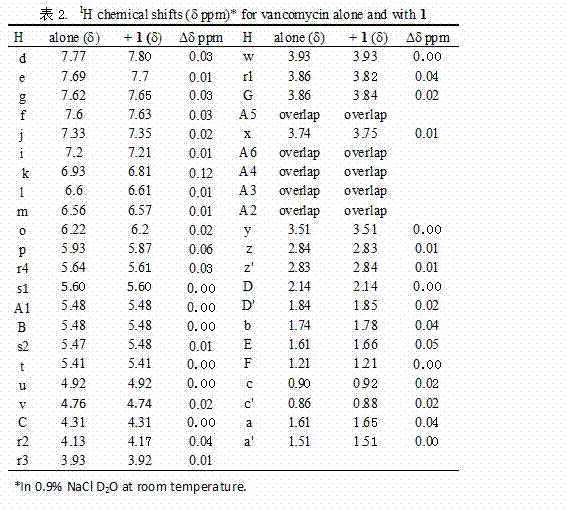
InactiveCN102755629AAntibacterial agentsPowder deliveryCyclodextrin DerivativesAntibacterial activity
The invention aims to provide a component of an inclusion compound capable of increasing the antibacterial activity of vancomycin and a preparation method. The inclusion compound is formed by taking a beta-cyclodextrin derivative, i.e., 6-[(L-phenylalanyl)-amino]-beta-cyclodextrin as a host compound and taking vancomycin as a guest compound. As proved by an in-vitro antibacterial activity experiment of the inclusion compound, vancomycin has the efficacy-enhancing effect of 4-8 times of minimum bacteriostasis solubility on the antibacterial activities of multiple bacterium strains, particularly methicillin resistant staphylococcus aureus (MRSA). The bacterium-resisting and efficacy-enhancing mechanisms of the inclusion compound formed by vancomycin and 6-[(L-phenylalanyl)-amino]-beta-cyclodextrin are explained by performing a 1HNMR (1h Nuclear Magnetic Resonance) experiment, analyzing the chemical structural conformation of the inclusion compound and combining the original biochemical acting mechanism of the antibacterial activity of vancomycin. The inclusion compound for increasing the antibacterial activity of vancomycin can be applied to antibacterial treatment of pathogenic bacteria which are insensitive to vancomycin or have low antibacterial activities. The component can be developed into a novel vancomycin synergistic agent for constituting a novel medicament together with vancomycin.
Owner:SHANGHAI RUICHUANG MEDICAL TECH
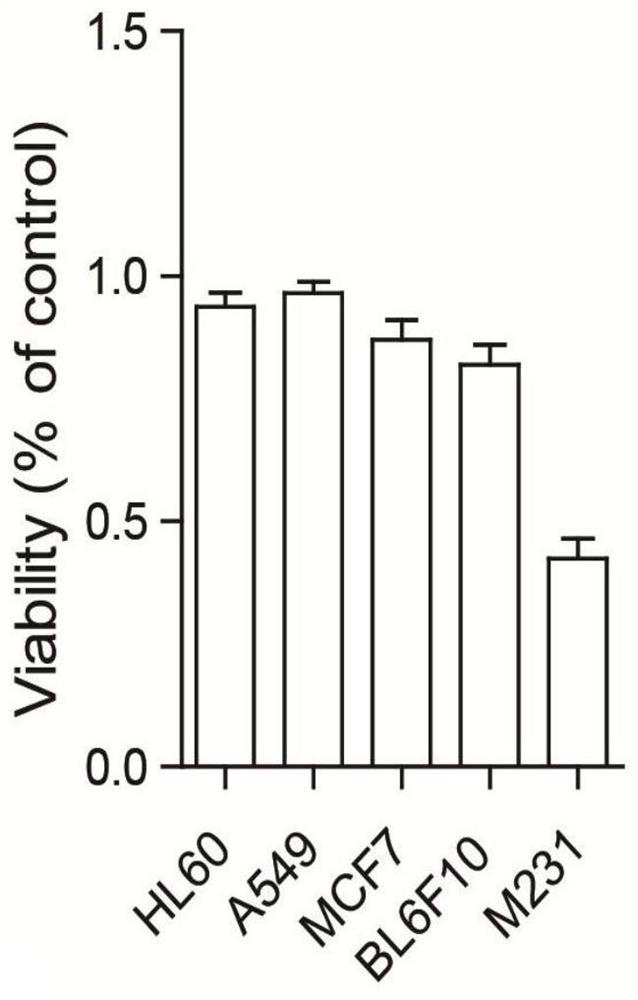
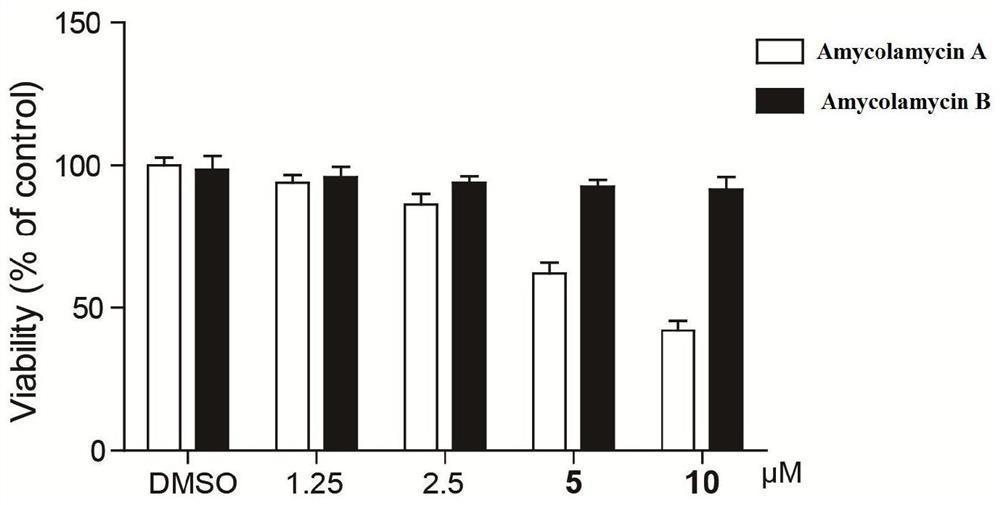
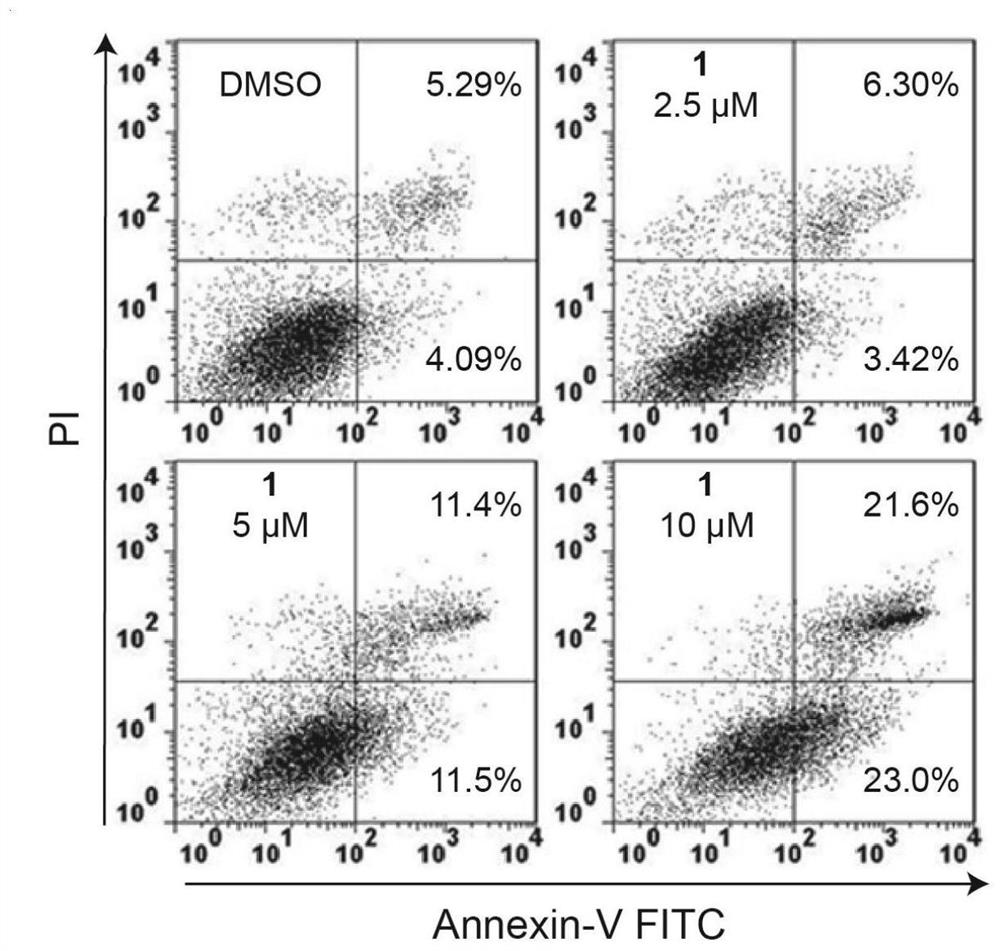
Emiclamycin and its preparation method and application
ActiveCN109678917BOvercoming Drug Resistance DisadvantagesHigh antibacterial activityAntibacterial agentsOrganic active ingredientsAntibiotic resistanceOncology
The invention discloses an emaclamycin and its preparation method and application. The said emaclamycin is derived from the liquid fermentation product of the endophytic actinomycete Amycolatopsis sp.HCa4 in the intestinal tract of migratory locust, including emaclamycin A or emaclamycin B. Experimental results show that emaclamycin has strong antibacterial activity against methicillin-resistant Staphylococcus aureus and strong cytotoxic activity against human breast cancer cell M231, and can be prepared into a new type of anti-tumor lead compound, to a certain extent Overcome the shortcomings of existing antibiotic resistance.
Owner:NANJING UNIV
Derivatives of ferruginous alkaloids and their application in the preparation of anti-drug-resistant bacteria
ActiveCN103980251BEnhanced inhibitory effectInhibition of reproductionAntibacterial agentsOrganic active ingredientsAmpicillinPharmaceutical Substances
Owner:GUANGDONG UNIV OF TECH
Polypeptide compound with broad-spectrum bactericidal activity on gram-positive bacteria
PendingCN113717261AHas broad-spectrum bactericidal activityAntibacterial agentsDepsipeptidesStaphyloccocus aureusAntibiotic drug
The invention discloses a polypeptide compound with broad-spectrum bactericidal activity on gram-positive bacteria. The polypeptide compound is formed by combining polypeptides with sequences shown in SEQ ID No.1 and SEQ ID No.2. The polypeptide compound with broad-spectrum bactericidal activity on gram-positive bacteria is derived from standard sensitive staphylococcus aureus (ATCC 29213). The polypeptide compound disclosed by the invention has broad-spectrum bactericidal activity on gram-positive bacteria and has a killing effect on methicillin-resistant bacteria and vancomycin-resistant bacteria which are difficultly killed by antibiotics.
Owner:NORTHWEST A & F UNIV
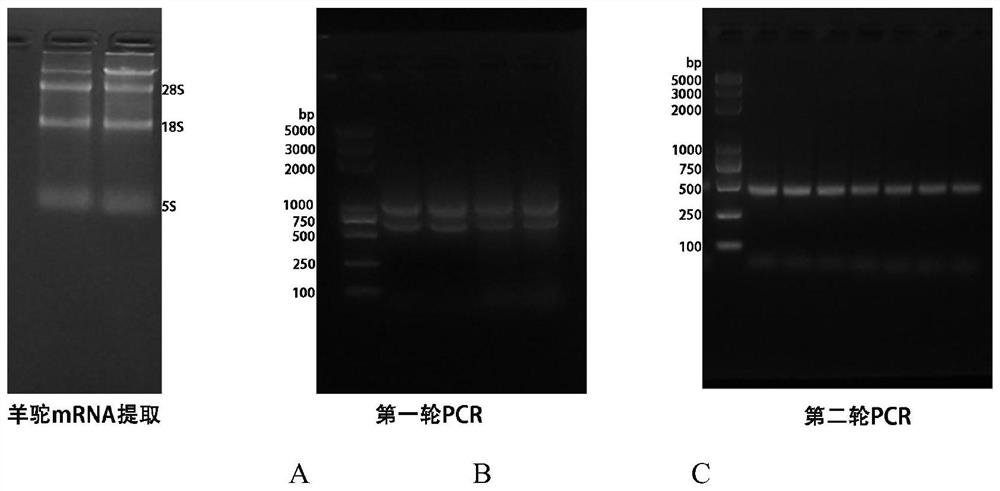
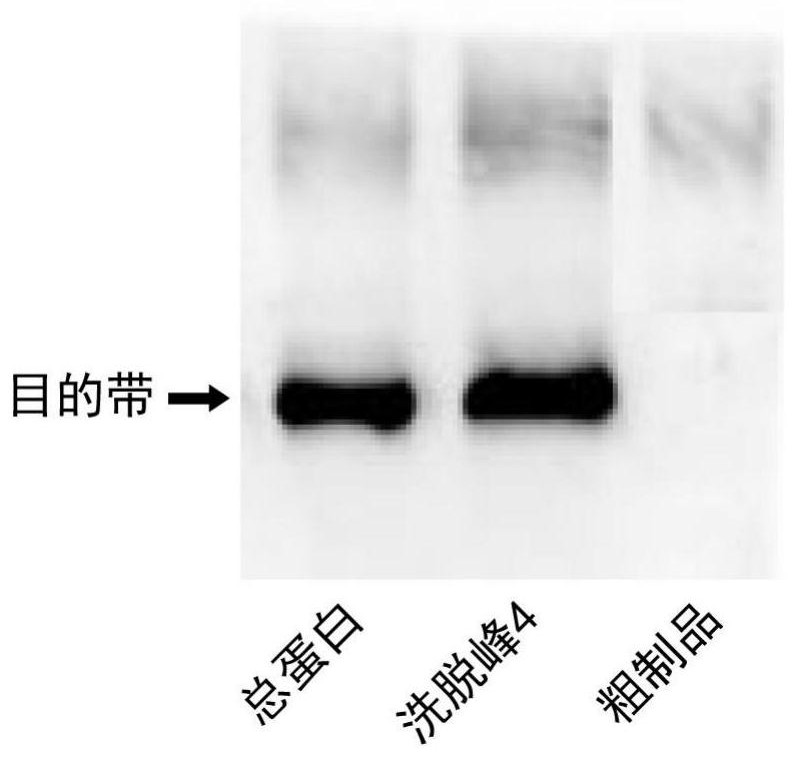
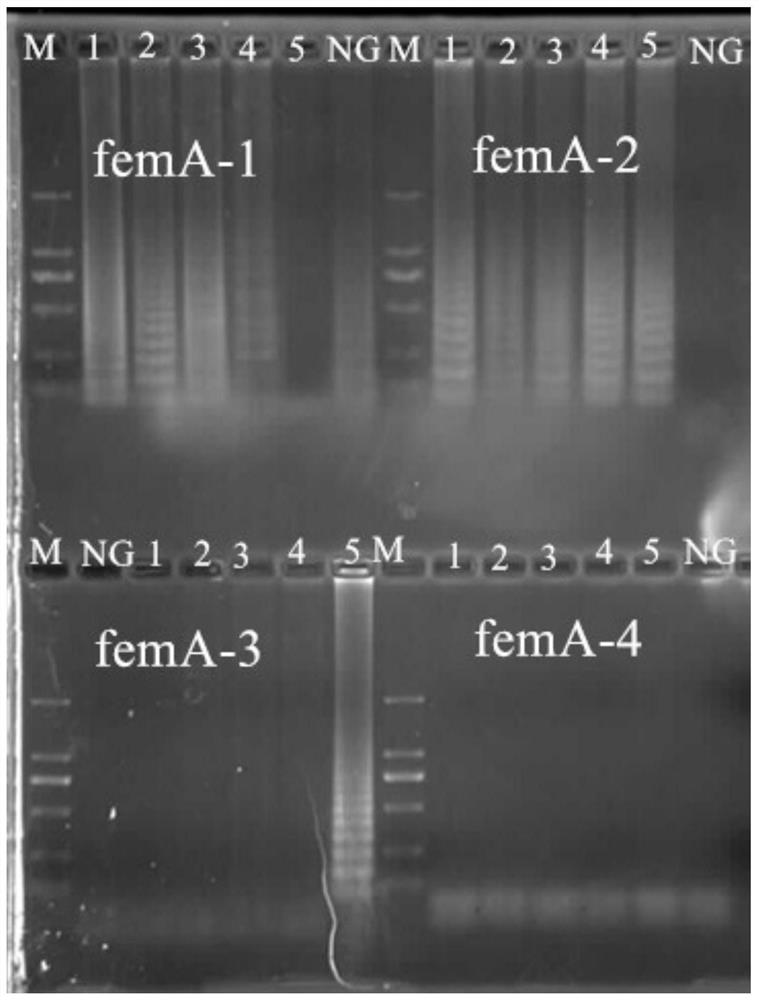
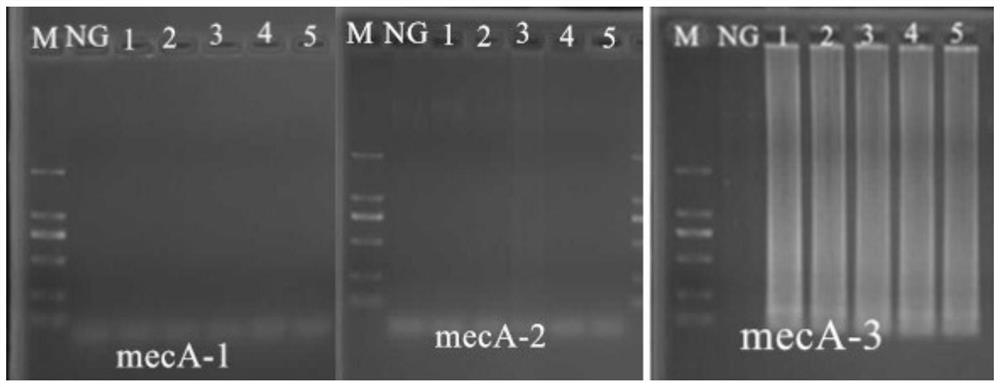
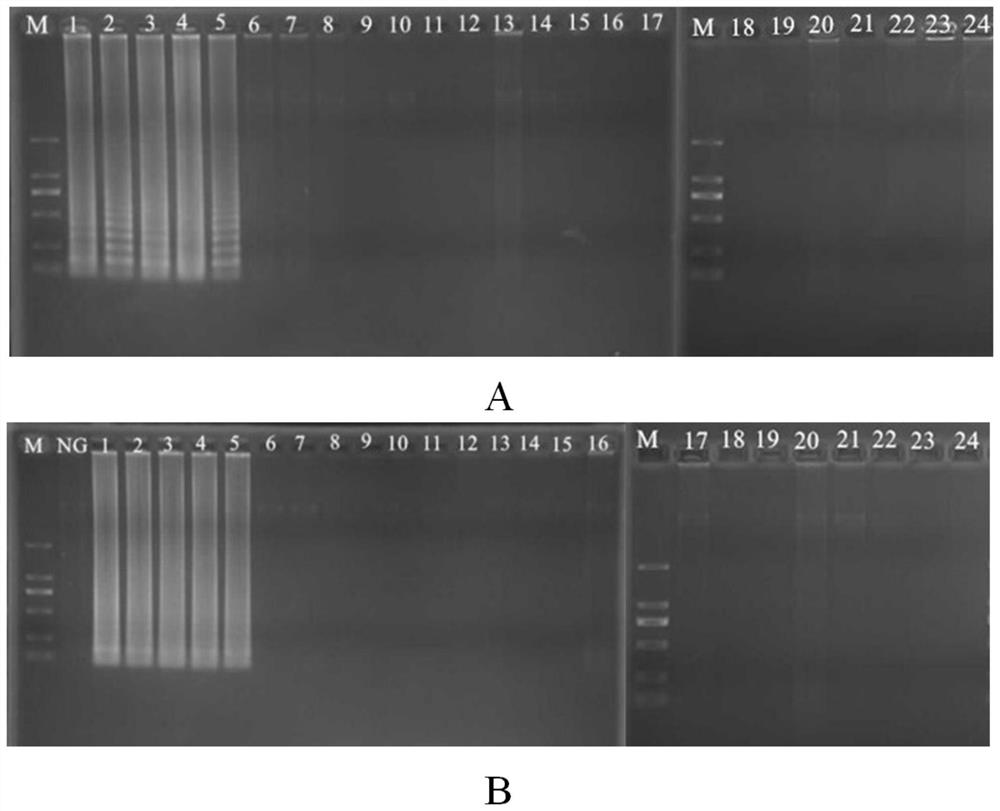
A kind of primer, kit and method for detecting methicillin-resistant staphylococcus aureus by PSR
ActiveCN109355403BQuick checkoutAccurate detectionMicrobiological testing/measurementMicroorganism based processesMethicillin resistant StaphylococcusStaphylococcus aureus
The invention discloses primers, kit and method for detecting methicillin-resistant Staphylococcus aureus by PSR (polymerase spiral reaction). The primers provided herein are shown as SEQ ID NO. 1 to4 and suitable for detecting methicillin-resistant Staphylococcus aureus by PSR; specificity, sensitivity and reliability can be detected for methicillin-resistant Staphylococcus aureus by detecting specific target sequences femA and mecA. The invention also provides a kit and method for detecting methicillin-resistant Staphylococcus aureus by PSR based on the primers; the method has the advantages of high sensitivity, good specificity, good operational convenience, high operational speed, accurate and reliable results, low detection cost and suitability to field detection; no special expensive instruments or agents are required; after developing with a fluorescent dye, the results can be judged with eyes; the kit and method are particularly suitable for detection in small- and medium-sized units and field detection and have a promising application prospect.
Owner:SOUTH CHINA UNIV OF TECH
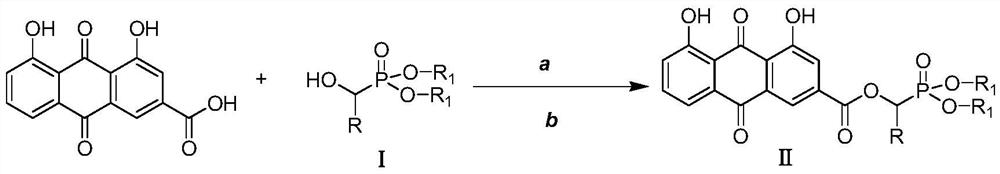
A kind of rhein ester derivative and its preparation method and application
The invention discloses a rhubarb acid ester derivative in the field of antibiotic compounds. The chemical structural formula of rhubarb acid ester is as shown in the formula (II), the formula (II) isshown in the description, wherein R is a chain substituent group of 1-5 carbon atoms or a naphthenic base or an aromatic hydrocarbon group of 3-6 carbon atoms. An antibiotic activity test proves thatthe rhubarb acid ester derivative with the novel structure has the antibiotic activity on methicillin-resistant staphylococcus aureus (MRSA). A plurality of targets have the outstanding antibiotic effect on methicillin-resistant staphylococcus aureus (MRSA), are superior to the reference drug oxacillin and is close to the reference drug vancomycin, and the rhubarb acid ester derivative can be served as a candidate compound to resist methicillin-resistant staphylococcus aureus (MRSA) and be researched.
Owner:ZUNYI MEDICAL UNIVERSITY
Use of zinc and copper gluconate in the treatment of methicillin-resistant Staphylococcus aureus
The present invention relates to the use of zinc and copper gluconate in the treatment of methicillin-resistant Staphylococcus aureus and dietary supplements for mammals comprising zinc and copper gluconate and their use in treatment of methicillin-resistant Staphylococcus aureus.
Owner:DANTRACE DANFEED IVS +1
Methods and compositions for suppressing virulence of methicillin resistant staphylococcus aureus
ActiveUS20170182145A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsStaphylococcus aureusVirulence
Owner:MICROVAX LLC
Method for detecting a methicillin resistant coagulase positive staphylococcus aureus strain
ActiveUS20140370512A1Reduce complexityEfficient and reliableSugar derivativesMicrobiological testing/measurementStaphylococcus aureusCoagulase test
The invention relates to a method for detection of a methicillin resistant coagulase positive Staphylococcus aureus (MRSA) strain by means of a sequence specific amplification reaction.
Owner:CONGEN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com