Component for forming bacterium-resisting and efficacy-enhancing inclusion compound together with vancomycin and preparation method and application thereof
A vancomycin, antibacterial and synergistic technology, applied in the direction of non-active components of polymer compounds, glycopeptide components, antibacterial drugs, etc., can solve the problem of decreased sensitivity of vancomycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
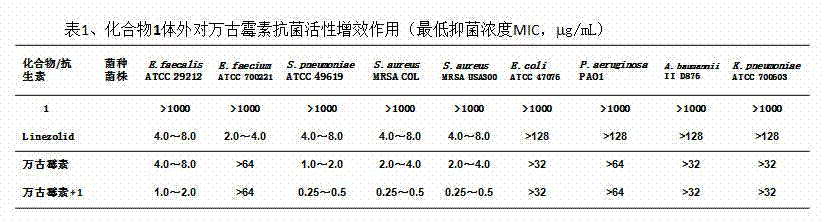
[0026] Vancomycin, 6-[(L-phenylalanyl)-amino]-β-cyclodextrin hydrochloride ( 1 ) and its inclusion complex in vitro antibacterial activity test
[0027] Test 6-[(L-phenylalanyl)-amino]-β-cyclodextrin hydrochloride ( 1 ), positive control drug Linezolid, vancomycin, 1:1 molar solubility of vancomycin: 6-[(L-phenylalanyl)-amino]-β-cyclodextrin hydrochloride ( 1 ) antibacterial activity against nine clinically common bacterial strains, the results are shown in figure 1 .
Embodiment 2
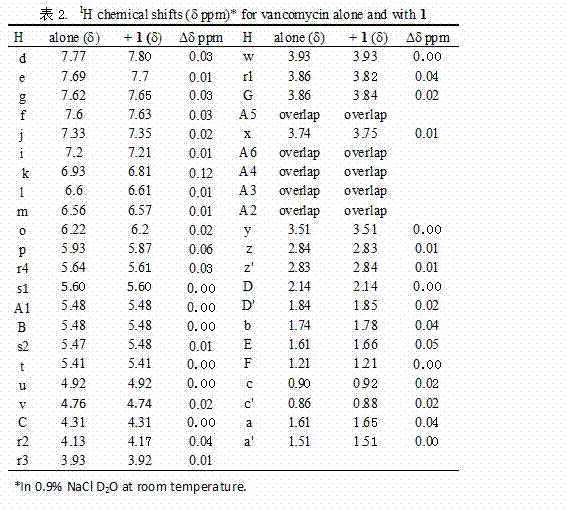
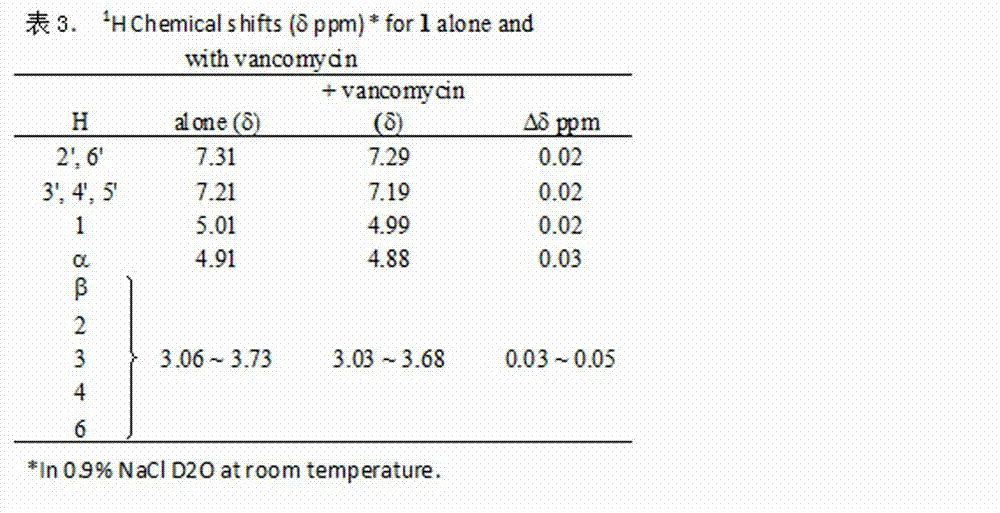
[0029]6-[(L-phenylalanyl)-amino]-β-cyclodextrin hydrochloride ( 1 ), vancomycin and clathrates 1 H NMR Spectrum, Chemical Conformation Analysis and Antibacterial Synergistic Mechanism
[0030] 6-[(L-phenylalanyl)-amino]-β-cyclodextrin hydrochloride ( 1 ) and vancomycin were directly prepared with 0.9% normal saline heavy water, the concentration was 0.016 moles, respectively for 1 H NMR determination; compound 1 Prepare with vancomycin at a molar ratio of 1:1 with 0.9% normal saline heavy water, the concentration is 0.016 molar, and carry out one hour after mixing 1 H NMR determination. See the test results figure 2 and 3 . 6-[(L-phenylalanyl)-amino]-β-cyclodextrin hydrochloride ( 1 ) and vancomycin, the chemical conformation analysis of the inclusion complex and its antibacterial synergistic mechanism are shown in Figure 4 , Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com