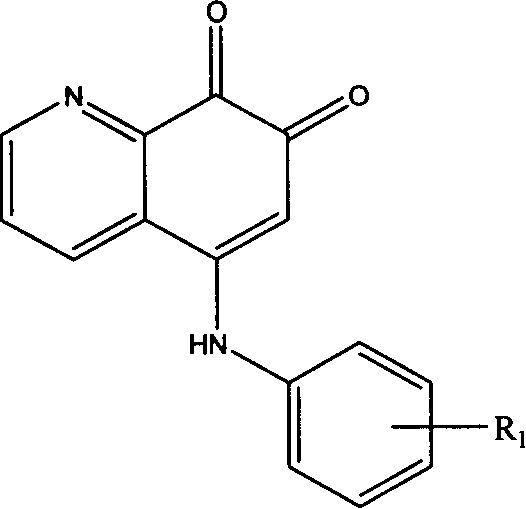
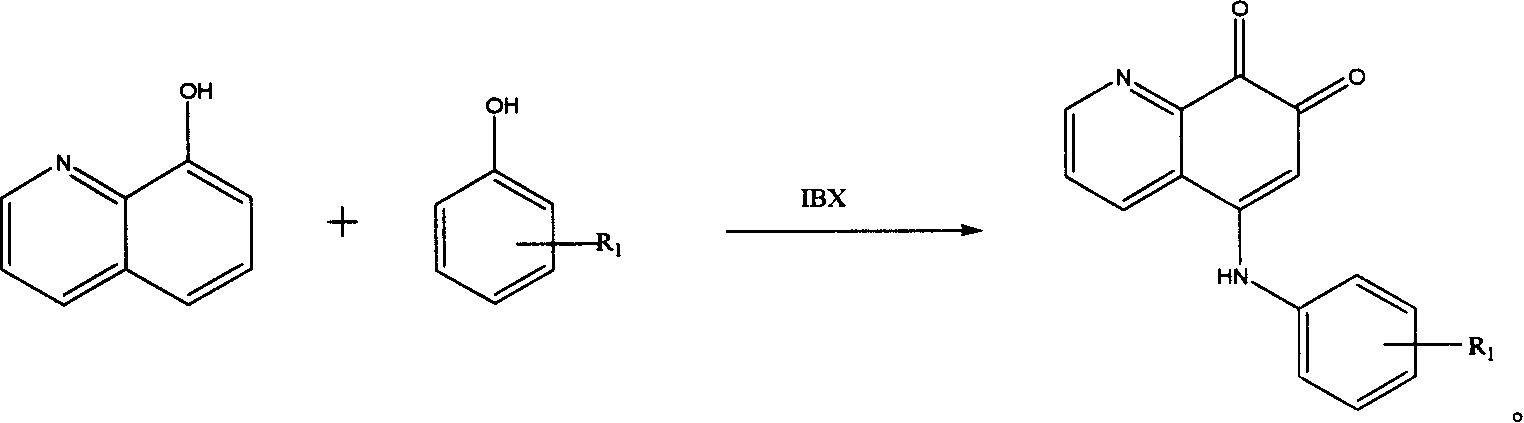
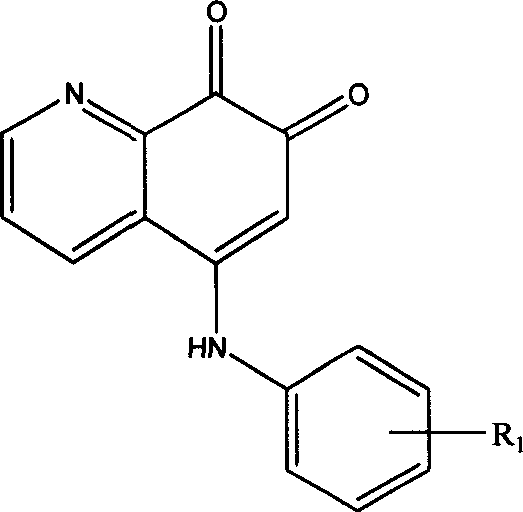
5-arylamino quinolyl-7,8-dione derivative and its application in preparing antibiotic medicine
A technology of arylaminoquinoline and antibacterial drugs, which is applied in the field of drugs against methicillin-resistant Staphylococcus aureus, and can solve problems such as lack of drug treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: OX-03
[0017] Dissolve 1.45g (10mmol) of 8-hydroxyquinoline and 1.08g (10mmol) of p-methylaniline in 20ml of DMF solution, add an appropriate amount of IBX, react at room temperature (25°C-30°C) for 2h, concentrate under reduced pressure and use 20ml Ethyl acetate was extracted three times, the solvent was evaporated to dryness, and silica gel column chromatography (CH3Cl:CH3OH: 7:3) was used to obtain 0.65 g of a brownish-red solid (about 40% yield). The structure of the product was determined by IR, MMR, MS and elemental analysis data.
Embodiment 2
[0018] Example 2: GX-09
[0019] 1.45g (10mmol) of 8-hydroxyquinoline and 1.24g (10mmol) of p-methoxyaniline were dissolved in 20ml of DMF solution, an appropriate amount of IBX was added, and reacted at room temperature (25°C-30°C) for 1h, concentrated under reduced pressure and used 20ml of ethyl acetate was extracted three times, the solvent was evaporated to dryness, and then silica gel column chromatography (CH3Cl:CH3OH ratio of 7:3) was used to obtain 1.4g of a brownish red solid (about 50% yield). The structure of the product was determined by IR, MMR, MS and elemental analysis data.
Embodiment 3
[0020] Embodiment 3: GX-10
[0021] 1.45g (10mmol) of 8-hydroxyquinoline and 1.72g (10mmol) of p-bromoaniline were dissolved in 20ml of DMF solution, an appropriate amount of IBX was added, and the reaction was carried out at room temperature (25°C-30°C) for 6h. Ethyl ester was extracted three times, the solvent was evaporated to dryness, and silica gel column chromatography (CH3Cl:CH3OH ratio 7:3) was used to obtain 0.82 g of a brownish-red solid (about 25% yield). The structure of the product was determined by IR, MMR, MS and elemental analysis data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com