Cyclic hexadepsipeptide compound desotamide A4 and application thereof in preparation of antibacterial drugs
A technology of antibacterial drugs and compounds, applied in the field of natural products, to achieve the effect of enhanced activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
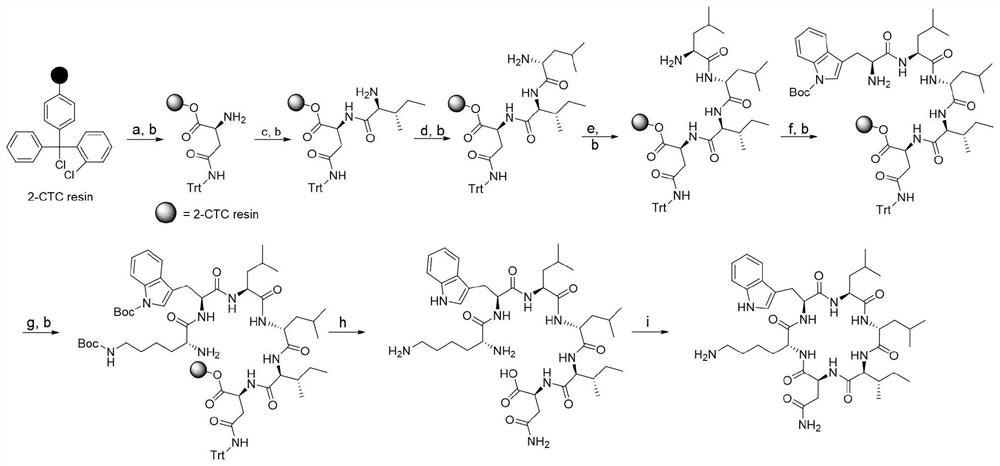
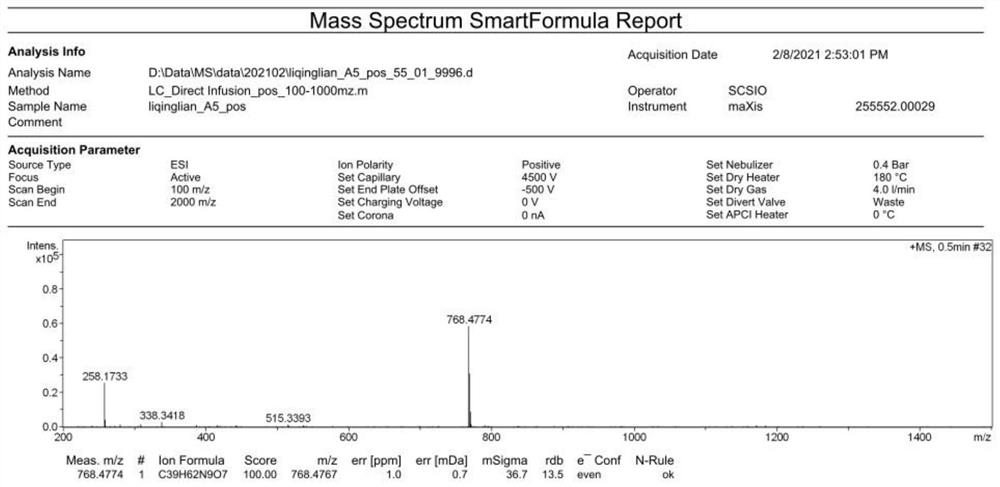
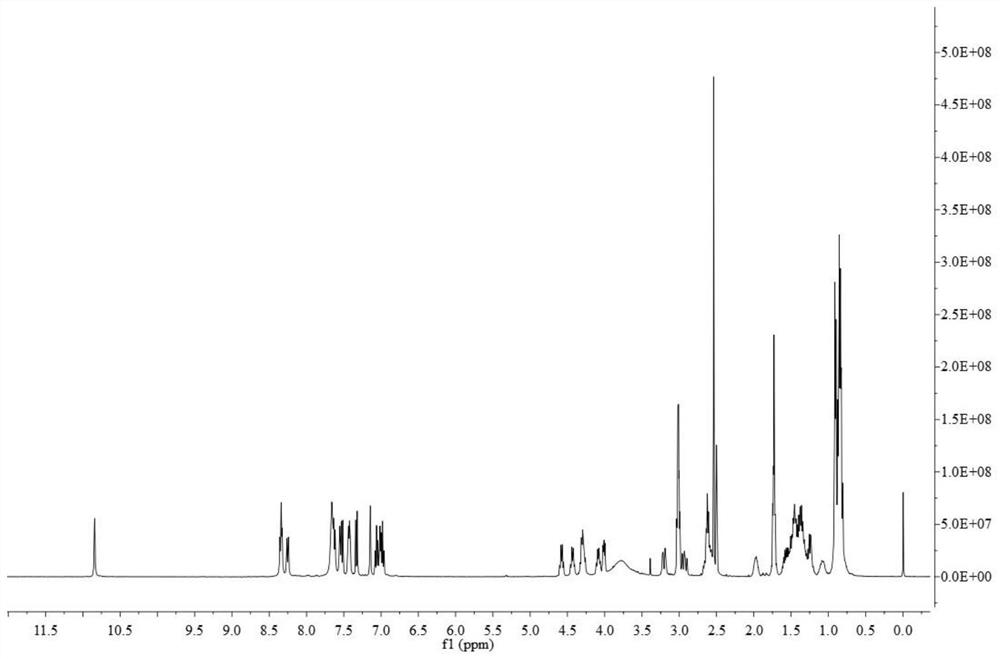
[0016] Example 1: Solid-phase chemical synthesis and structural confirmation of desotamide A4
[0017] Prepare desotamide A4 by solid-phase chemical synthesis method by Shanghai Ziyu Biotechnology Co., Ltd., the specific steps are as follows ( figure 1 ):
[0018] (1) Connecting to the first amino acid: Add 2-Cl-Trt resin to a dry and clean peptide reaction column, swell with N,N-dimethylformamide (DMF) for 0.5 hours, wash and then drain. Add the first amino acid raw material Fomc-L-Asn, O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU), N,N-diisopropylethylamine (DIEA), DMF as a solvent, React for 1.5 hours.
[0019] (2) Blocking: Add methanol directly after the reaction, so that the resin does not agglomerate, and react for 30 minutes. Wash with dichloromethane (DCM) and DMF (15 mL each for 3 times, 1 minute each), and drain.
[0020] (3) Removal: add 10 mL of DMF solution containing 20% (volume fraction) piperidine, blow with nitrogen for 15 minutes, remov...
Embodiment 2
[0031] Embodiment 2: Antibacterial activity test analysis of compound desotamides A4 to series of Gram-positive pathogenic bacteria
[0032] The inhibitory activity of the compound desotamides A4 against a series of Staphylococcus aureus was tested by microwell method. A series of Staphylococcus aureus was cultured in Mueller-Hinton (MH) broth medium. And prepare the sample solution before the experimental bacteria grow well. Concentrations of samples and positive controls were configured, and ampicillin and vancomycin were selected as positive controls. All samples were configured at 3200 μg / mL and dissolved in DMSO. Add 92 μL of sterile MH broth to the first column of the 96-well plate with a row gun, and add 50 μL of MH broth to the remaining columns, mark and cover for later use. Pipette 8 μL (the initial concentration is 128 μg / mL; if the initial concentration is 64 μg / mL, take 4 μL; if the initial concentration is 32 μg / mL, take 2 μL) and add the prepared sample or po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com