Anti-D-dimer monoclonal antibody and preparation method thereof
A monoclonal antibody and dimer technology, applied in the biological field, can solve the problems of unfavorable industrial production of anti-D-dimer antibody, poor stability between batches, complicated production process, etc., to achieve control stability and improve The effect of expression amount and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Screening of anti-D-dimer monoclonal antibodies
[0023] 1. Animal immunity
[0024] Take female 6-week-old BALB / c mice, and inject pure D-dimer with a concentration of 6 mg / mL for the first immunization, and inject it into the abdominal cavity and under the armpit of the limbs, with a total amount of 1 mL; every 2 weeks, the same method is used to boost Immunize once and immunize three times in total. On the 4th day after the last immunization, blood was collected from the eyeballs of mice after three immunizations, and the serum was centrifuged to separate the mice with high titer by ELISA method to prepare for fusion.
[0025] 2. Preparation of hybridoma cell lines
[0026] Take the splenocytes of mice that have completed the immunization process and fuse them with mouse myeloma cell SP2 / 0, and prepare feeder cells one day before fusion; take mouse splenocytes under aseptic conditions during fusion, and mix them with SP2 / 0 cells at a ratio of 10:1 Mix, m...
Embodiment 2
[0036] Example 2: Anti-D-dimer monoclonal antibody hybridoma expression
[0037] Hybridoma cells were cultured at 0.6×10 with CD Hybridoma (Gibco) medium 6 The inoculation density of cells / mL was diluted into a 3L cell culture reactor, and the initial culture volume was 1L; the stirring speed was set to 200r / min; the pH was set to 7.00, DeadBand was set to 0.10, and the associated sodium bicarbonate solution and CO 2 pH adjustment was performed; the dissolved oxygen was set to 40% (dissolved oxygen in saturated air was 100%), and the culture temperature was set to 36.5°C. On the 3rd, 5th, 7th, 9th, and 11th day, the feeding medium was used for feeding, and the feeding volume of the feeding medium Feed B Plus (Gibco) was 3% of the initial culture volume.
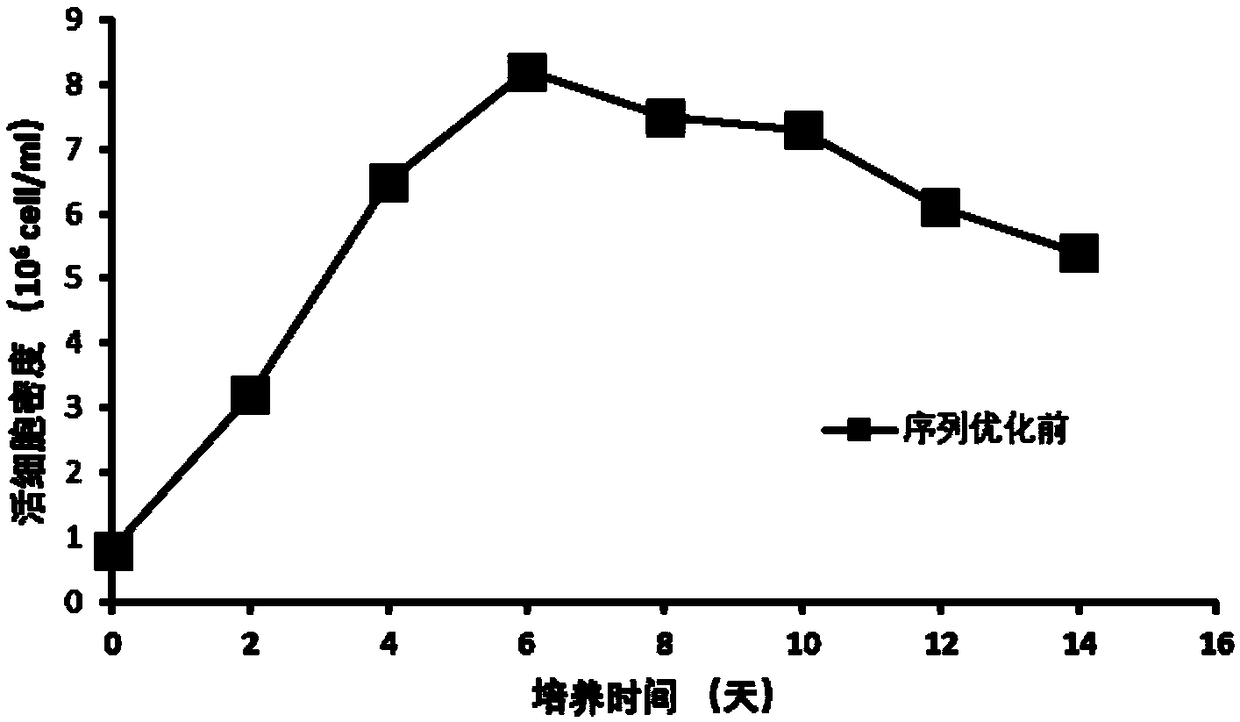
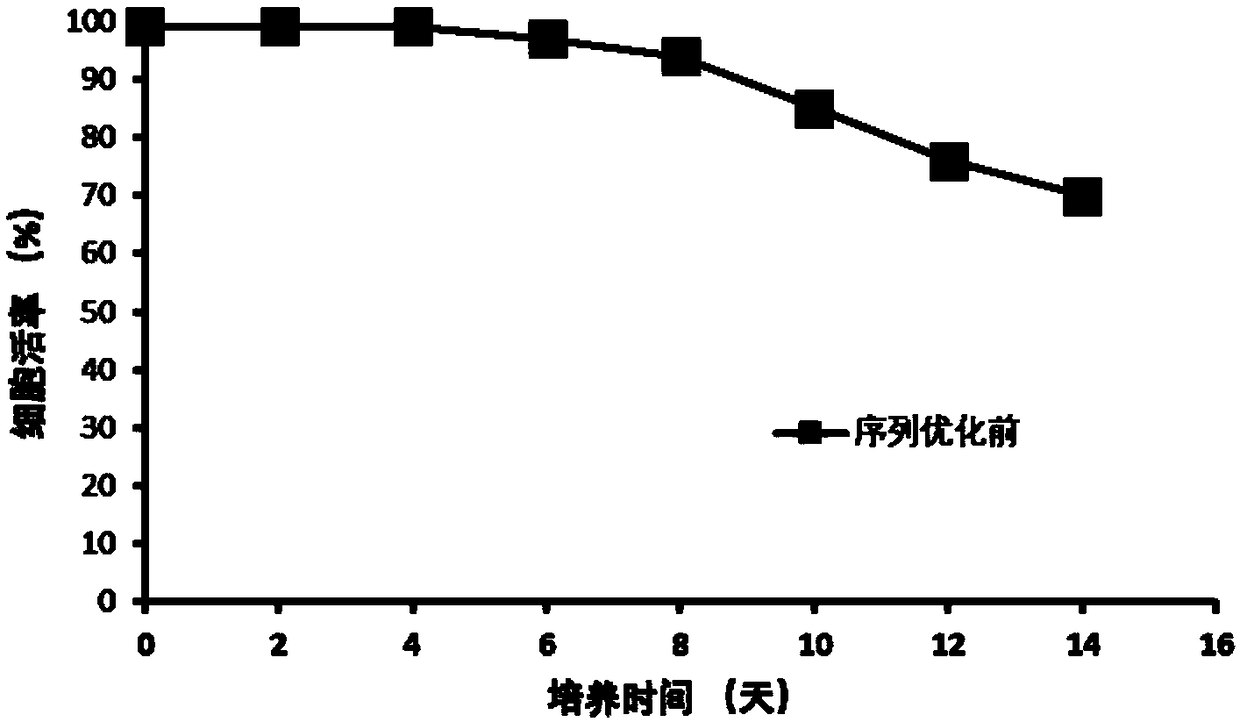
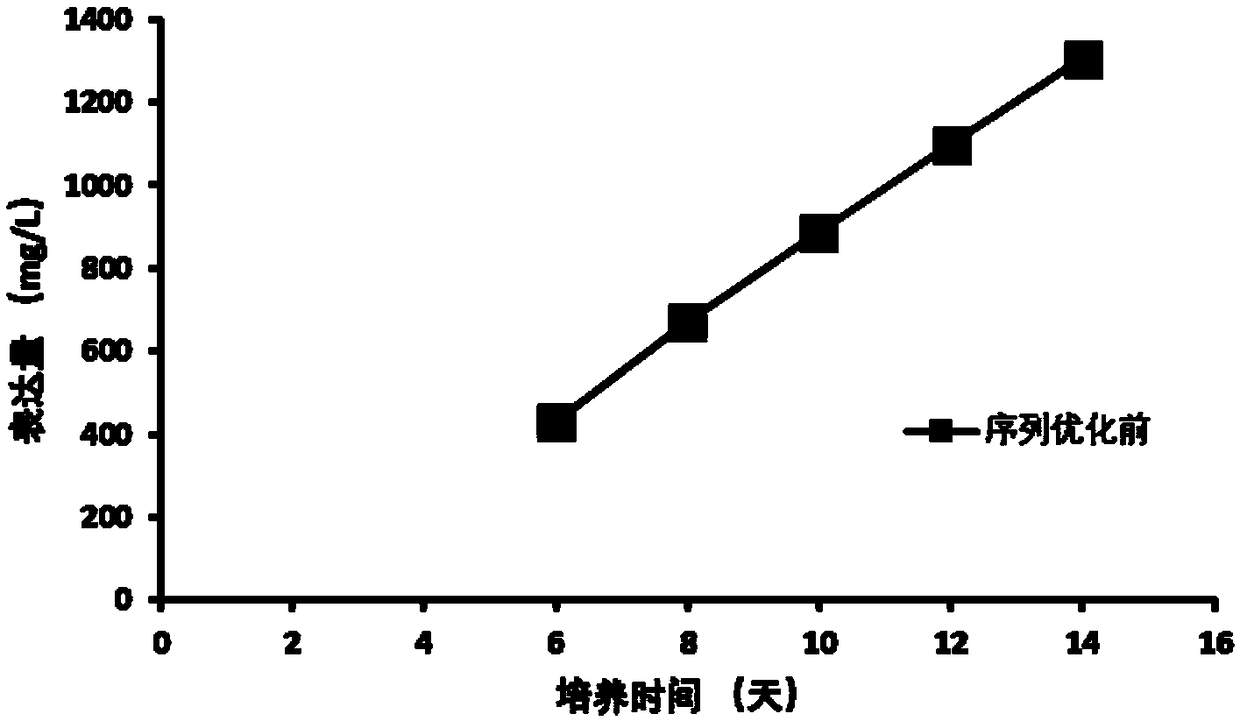
[0038] The maximum cell density reaches 8×10 6 cells / mL( figure 1 ), the cell viability was 70% at the end of the culture ( figure 2 ), the final expression level of anti-D-dimer monoclonal antibody reached 406mg / L ( ima...
Embodiment 3
[0040] Embodiment 3: Anti-D-dimer monoclonal antibody sequence optimization
[0041] The sequence of the constant region of the anti-D-dimer monoclonal antibody is replaced with a highly expressed sequence. The light chain sequence of the optimized complete monoclonal antibody is shown as SEQ ID NO.5, and the heavy chain sequence is shown as SEQ ID NO.6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com