An extraction and separation process for the co-production of pure europium and pure erbium by fractional distillation and extraction
An extraction and process technology, applied in the field of extraction and separation process, to achieve the effect of reducing acid and alkali consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The volume percentage of C272 in the kerosene solution in which the organic phase of the saponified C272 is the extraction agent C272 is 30%, and the saponification rate is 36%.
[0034] The pH of the europium chloride-rich solution is 3, and the rare earth element concentrations are: Pr 0.030g / L, Nd 0.30g / L, Sm40.0g / L, Eu 80.0g / L, Gd 30.0g / L, Tb 1.0g / L L, Dy 0.30g / L.
[0035] The pH of erbium-rich chloride is 3, and the rare earth element concentrations are: Gd 0.0030g / L, Tb 0.050g / L, Dy 0.30g / L, Ho 35.0g / L, Y 0.030g / L, Er 90.0g / L , Tm 25.0g / L, Yb 0.20g / L, Lu 0.10g / L.
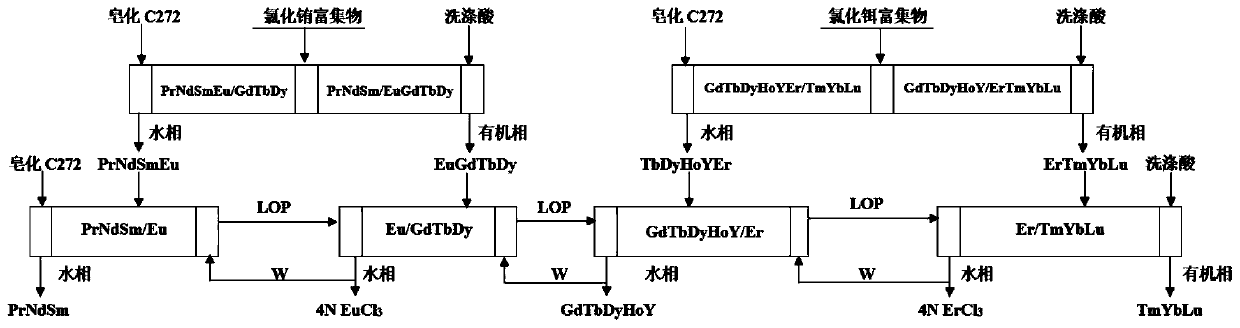
[0036] Step 1, fractional distillation and extraction to separate PrNdSmEu / EuGdTbDy:
[0037] The saponified C272 organic phase was used as the extraction organic phase, the europium-rich chloride solution was used as the first feed solution, and 3.0 mol / L HCl was used as the washing acid. The organic phase of saponified C272 enters the PrNdSmEu / EuGdTbDy fractionation extraction system from the first...
Embodiment 2
[0052] The volume percentage of C272 in the kerosene solution in which the organic phase of the saponified C272 is the extraction agent C272 is 30%, and the saponification rate is 36%.
[0053] The pH of the europium chloride-rich solution is 2, and the rare earth element concentrations are: Pr 0.050g / L, Nd 0.50g / L, Sm60.0g / L, Eu 60.0g / L, Gd 50.0g / L, Tb 2.0g / L L, Dy 0.50g / L.
[0054] The pH of erbium chloride rich is 4, and the rare earth element concentrations are: Gd 0.0050g / L, Tb 0.10g / L, Dy 0.50g / L, Ho 50.0g / L, Y 0.050g / L, Er 70.0g / L , Tm 35.0g / L, Yb 0.50g / L, Lu 0.30g / L.
[0055] Step 1, fractional distillation and extraction to separate PrNdSmEu / EuGdTbDy:
[0056] The saponified C272 organic phase was used as the extraction organic phase, the europium-rich chloride solution was used as the first feed solution, and 3.0 mol / L HCl was used as the washing acid. The organic phase of saponified C272 enters the PrNdSmEu / EuGdTbDy fractionation extraction system from the first ...
Embodiment 3
[0071] The volume percentage of C272 in the kerosene solution in which the organic phase of the saponified C272 is the extraction agent C272 is 30%, and the saponification rate is 36%.
[0072] The pH of the europium chloride-rich solution is 4, and the rare earth element concentrations are: Pr 0.010g / L, Nd 0.10g / L, Sm20.0g / L, Eu 100.0g / L, Gd 10.0g / L, Tb 0.50g / L L, Dy 0.10g / L.
[0073] The pH of erbium-rich chloride is 2, and the rare earth element concentrations are: Gd 0.0010g / L, Tb 0.030g / L, Dy 0.10g / L, Ho 20.0g / L, Y 0.010g / L, Er 110.0g / L , Tm 15.0g / L, Yb 0.10g / L, Lu 0.050g / L.
[0074] Step 1, fractional distillation and extraction to separate PrNdSmEu / EuGdTbDy:
[0075] The saponified C272 organic phase was used as the extraction organic phase, the europium-rich chloride solution was used as the first feed solution, and 3.0 mol / L HCl was used as the washing acid. The organic phase of saponified C272 enters the PrNdSmEu / EuGdTbDy fractionation extraction system from the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com