Oral pharmaceutical composition comprising sargrelate or a salt thereof
A composition and drug technology, applied in the direction of drug delivery, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of patients' medication compliance, improve or treat ischemic symptoms, and prevent treatment period increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] Patent Document 2 discloses a method for preparing the above-mentioned crystal form. In a specific example, preferably, the above-mentioned pharmaceutical composition contains about 30% of (±)-2-[2-(3-carboxypropionyloxy)-3-dimethylaminopropoxy]-3′ - Crystalline form II of methoxybibenzyl hydrochloride, more specifically, comprising more than about 70%.
[0023] In a specific example, the above-mentioned pharmaceutical composition may comprise about 95% or more or 98% or more of (±)-2-[2-(3-carboxypropionyloxy)-3-dimethylaminopropoxy] - Crystalline form II of 3'-methoxybibenzyl hydrochloride.
[0024] The above-mentioned active ingredients can be present in the above-mentioned immediate-release layer and the above-mentioned sustained-release layer in any proportion. It is present in a weight ratio of 1:1 to 3, and more specifically, can be present in a weight ratio of about 1:2 to 2.5. In a specific example, the immediate release layer may comprise 100 mg of (±)-2-[2...
Embodiment 1-10
[0064] Example 1-10: (±)-2-[2-(3-carboxypropionyloxy)-3-dimethylaminopropoxy]-3′-methoxybibenzyl hydrochloride sustained-release preparation of tablet
[0065] According to the composition and content of the sustained-release layer shown in Table 1 below, a binding solution in which povidone was dissolved in absolute ethanol was prepared, respectively, and (±)-2-[2-(3-carboxypropionyloxy) was mixed with -3-dimethylaminopropoxy]-3'-methoxybibenzyl hydrochloride, lactose hydrate, hydroxypropyl methylcellulose carbomer 941 and sodium starch glycolate, combined with the above After the solutions were combined, granulated and dried together, the sustained-release layer granules were prepared by sieving and granulating using a No. 20 sieve. Then, the sustained-release layer granules prepared above are post-mixed with magnesium stearate. The above granules were compressed at a hardness of 14 kgf to prepare sustained-release tablets. Then, the Opadry white film coating of Table 2 b...
experiment example 1
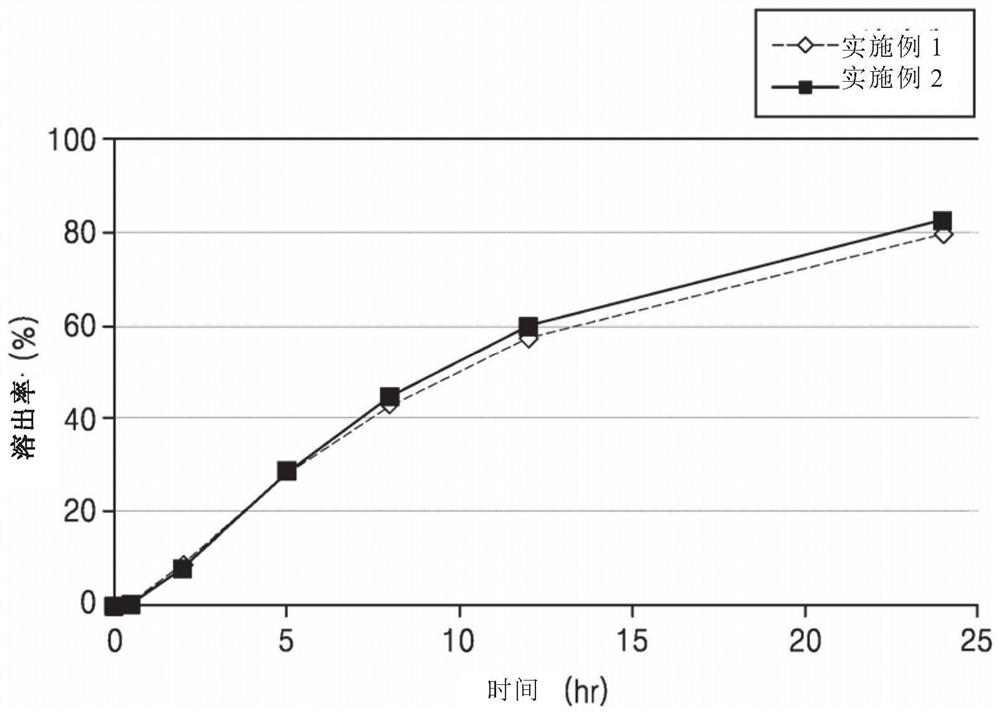
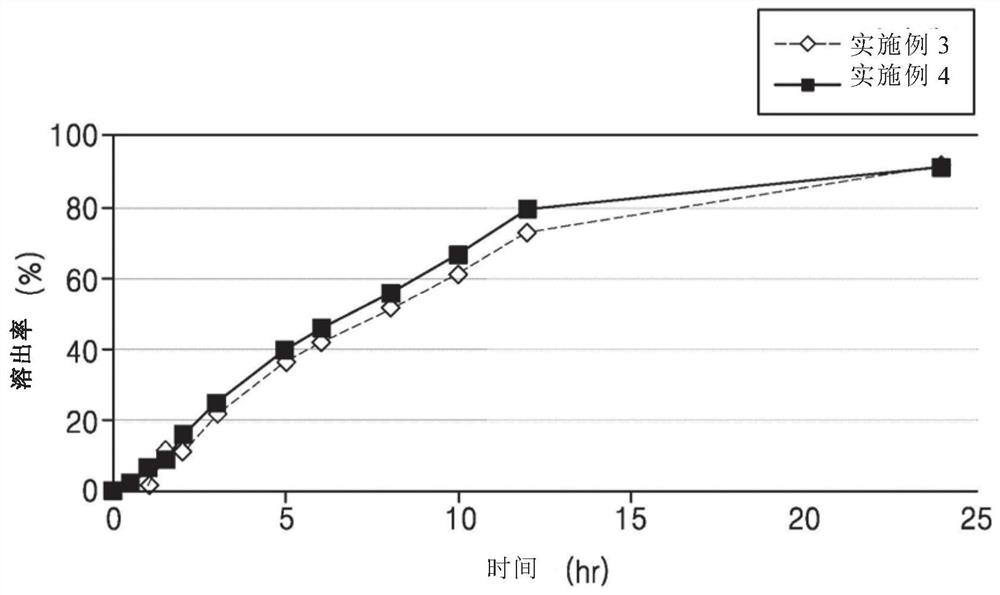
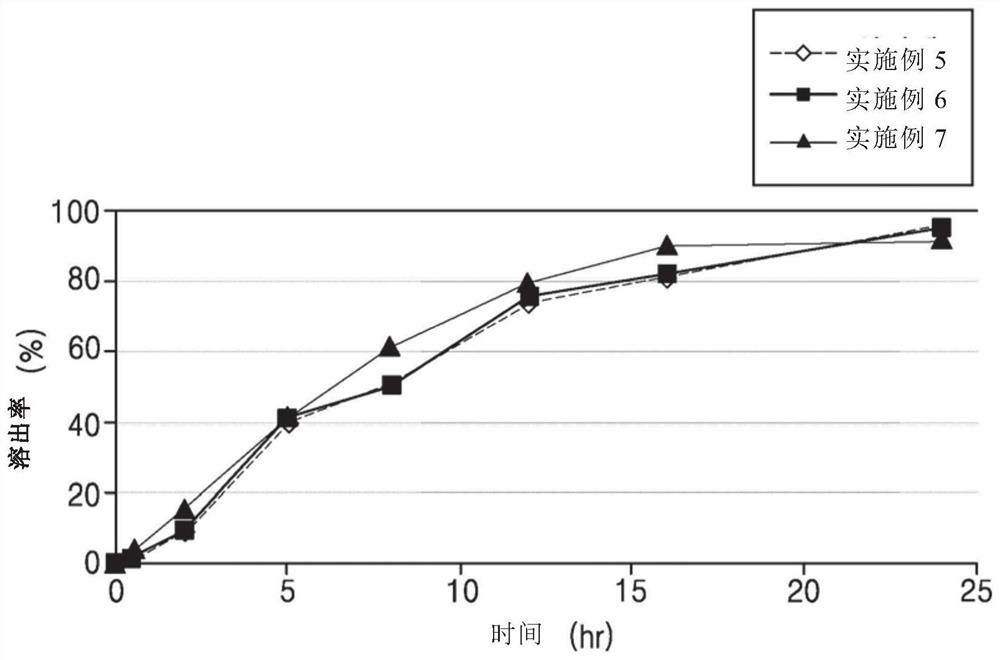
[0069] Experimental Example 1: Sustained release containing (±)-2-[2-(3-carboxypropionyloxy)-3-dimethylaminopropoxy]-3′-methoxybibenzyl hydrochloride Dissolution test of tablet (1)
[0070] (1) Prepare test solution
[0071] Dissolution conditions
[0072] Experimental device: The second method (paddle method) in the general experimental method of the Korean Pharmacopoeia
[0073] Dissolution: 900mL of water
[0074] Stirring speed: 50rpm
[0075] Test solution temperature: 37±0.5℃
[0076] According to the second method (paddle method) of the dissolution test method in the general test method of the Korean Pharmacopoeia, the dissolution test was carried out on each of the six sustained-release tablets prepared in the above Examples 1-10 under the above conditions. Filter through a syringe filter and discard 5 mL of the first filtrate, and use the subsequent filtrate as the test solution.
[0077] (2) Prepare standard solution
[0078] The standard product of (±)-2-[2-(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com