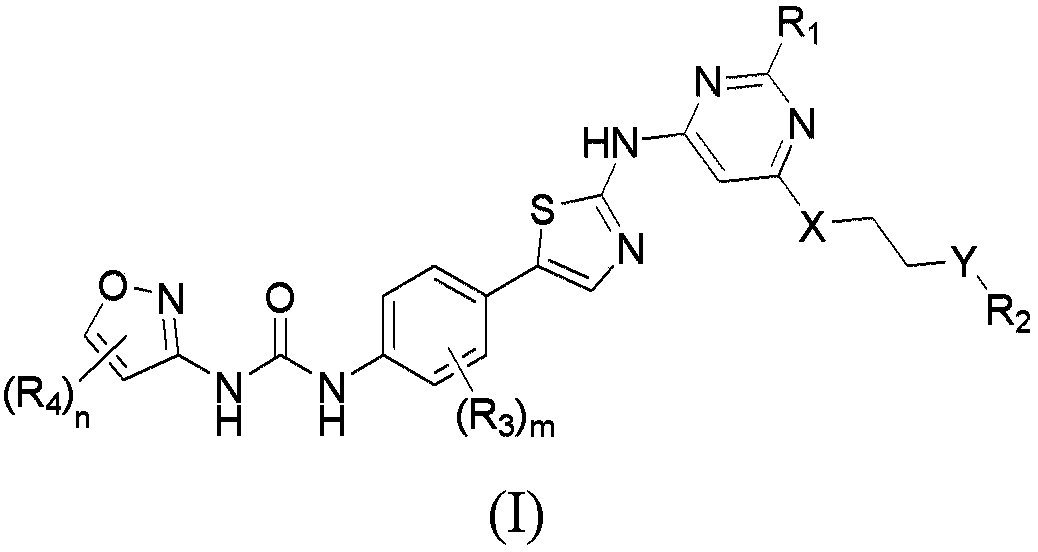
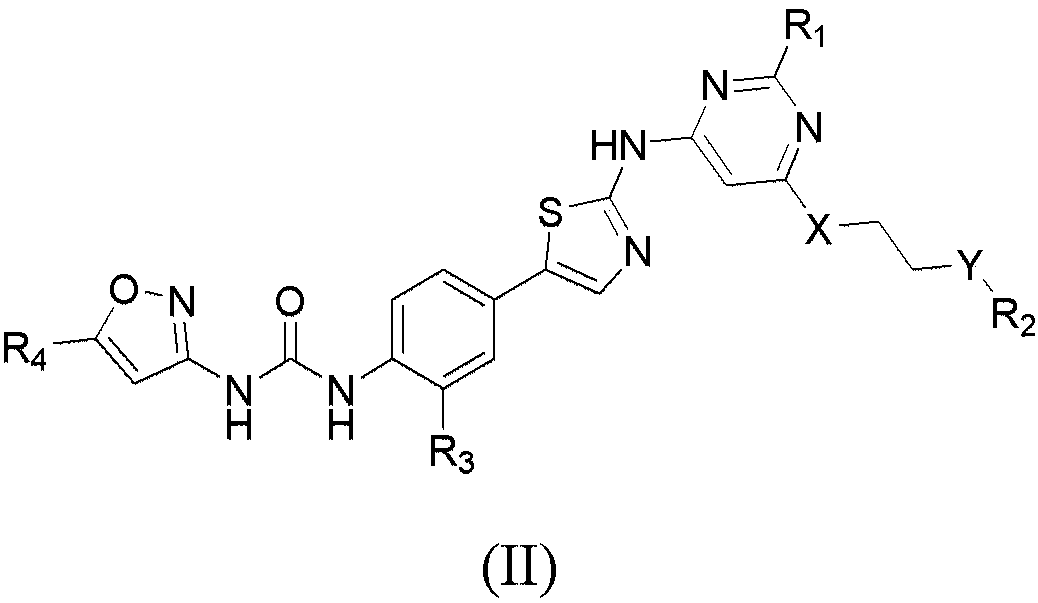
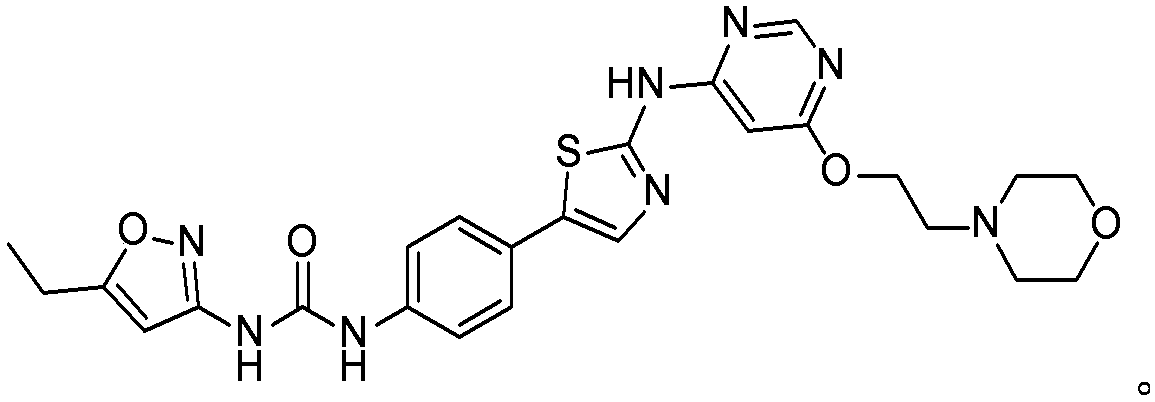
Aminothiazole compounds and use thereof
A compound and amino technology, applied in the field of aminothiazole compounds and their uses, can solve the problems of poor kinase selectivity, animal death and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of compound 1
[0054] Compound 1 was prepared according to the synthetic method described in Chen et al., European Journal of Medicinal Chemistry, 2015, 100, 151-161.
[0055] All chemicals and solvents were purchased from commercial suppliers and used as received. All reactions were performed under dry nitrogen atmosphere. The reaction was monitored by TLC using a Merck 60 F254 silica gel glass backplate (5×10 em); and each was detected visually under UV light irradiation (254 nm) or by spraying phosphomolybdic acid reagent (Aldrich) followed by heating at 80° C. area. All flash column chromatography was performed using Merck Kieselgel 60, No. 9385, 230-400 mesh ASTM silica gel as stationary phase. Proton ( 1 H) The nuclear magnetic resonance spectrum is measured with a Varian Mercury-300 or Varian Mercury-400 spectrometer. Chemical shifts are reported in parts per million (ppm) on a scale relative to the delta of the solvent peak reso...
Embodiment 2
[0061] 1-(5-Ethyl iso Azol-3-yl)-3-(4-(2-((6-(4-ethylpiperazin-1-yl)pyrimidin-4-yl)amino)thiazol-5-yl)phenyl)urea ( Hydrochloride)
[0062]
[0063] 1 H NMR (400MHz, DMSO-d6): δ11.14(s, 1H), 9.78(s, 1H), 9.62(s, 1H), 8.48(s, 1H), 7.74(s, 1H), 7.50(q , J=8.8Hz, 4H), 6.55(s, 1H), 6.38(s, 1H), 4.34(d, J=14.0Hz, 2H), 3.55(d, J=11.6Hz, 2H), 3.44-3.38 (m, 2H), 3.14-3.08(m, 2H), 3.00(q, J=9.6Hz, 2H), 2.69(q, J=7.6Hz, 2H), 1.26(t, J=7.6Hz, 3H) , 1.19(t, J=7.6Hz, 3H); MS(ES + )m / z(C 25 h 29 N 9 o 2 S) Calculated value: 519.22; Actual value: 520.2 (M+H + ).
Embodiment 3
[0065] 1-(5-Ethyl iso Azol-3-yl)-3-(4-(2-((6-(2-morpholineethoxy)pyrimidin-4-yl)amino)thiazol-5-yl)phenyl)urea (hydrochloride )
[0066]
[0067] 1 H NMR (400MHz, DMSO-d6): δ11.08(s, 1H), 9.75(s, 1H), 9.56(s, 1H), 8.62(s, 1H), 7.73(s, 1H), 7.50(q , J=8.8Hz, 4H), 6.54(s, 1H), 6.50(s, 1H), 4.71(s, 2H), 3.95(d, J=11.6Hz, 2H), 3.79(t, J=12.4Hz , 2H), 3.56(s, 2H), 3.47(d, J=12.4Hz, 2H), 3.15(d, J=10.0Hz, 2H), 2.69(q, J=7.6Hz, 2H), 1.19(t , J=7.6Hz, 3H); MS(ES + )m / z(C 25 h 28 N 8 o 4 S) Calculated value: 536.20; Actual value: 537.2 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com