Uses of bicyclol and pharmaceutically acceptable derivative thereof in prevention or treatment of drug-induced liver damage
A therapeutic drug, bicyclol technology, applied in the prevention or treatment of drug-induced liver injury, bicyclol and its pharmaceutically acceptable derivatives, can solve the problems of liver injury, AST and ALT, and reduce serum ALT and AST, copy The effect of increasing the number, preventing or treating drug-induced liver injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Animal experiments on mice for preventing or treating drug-induced liver injury caused by azithromycin with bicyclol
[0034] 80 healthy male KM mice (provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., certificate number: SCXK (Beijing) 2012-0001) were randomly divided into 8 groups, namely blank control group, azithromycin liver injury Model control group (model control group), 100mg / kg bicyclol prevention group (low dose prevention group), 200mg / kg bicyclol prevention group (medium dose prevention group), 300mg / kg bicyclol prevention group (high dose prevention group) , 100mg / kg bicyclol treatment group (low dose treatment group), 200mg / kg bicyclol treatment group (middle dose treatment group), 300mg / kg bicyclol treatment group (high dose treatment group), 10 in every group.
[0035] The mice in the blank control group had a normal diet throughout the experiment, and were not given azithromycin and bicyclol. After the experiment, th...
Embodiment 2
[0042] Embodiment 2: the mouse animal experiment of embodiment 1 keeps the detection of blood sample and liver tissue sample
[0043] ALT, AST, MDA, GSH, CAT, SOD, NO, NOS, ATPase were measured with corresponding kits produced and sold by Nanjing Jiancheng Bioengineering Research Institute; DNA and RNA were extracted with kits produced and sold by Dalian Bao Biological Company; ELISA kits were used to measure serum inflammatory factors IL-1β and TNF-α; liver tissue samples were made into pathological sections to observe the morphological changes. YBRGreen MIX was purchased from Nanjing Aibimeng Company.
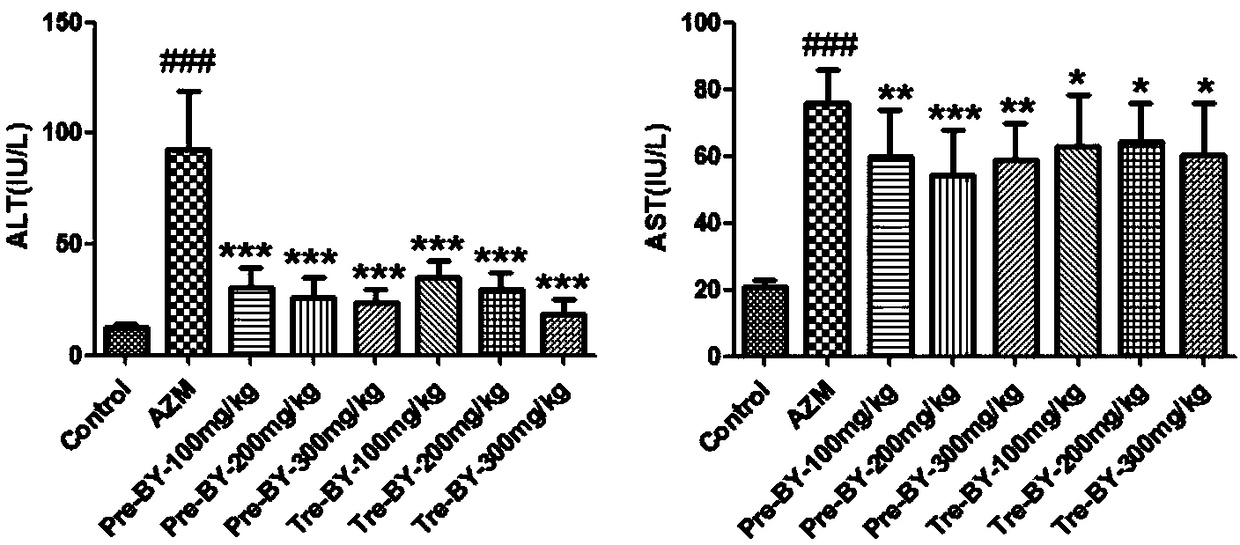
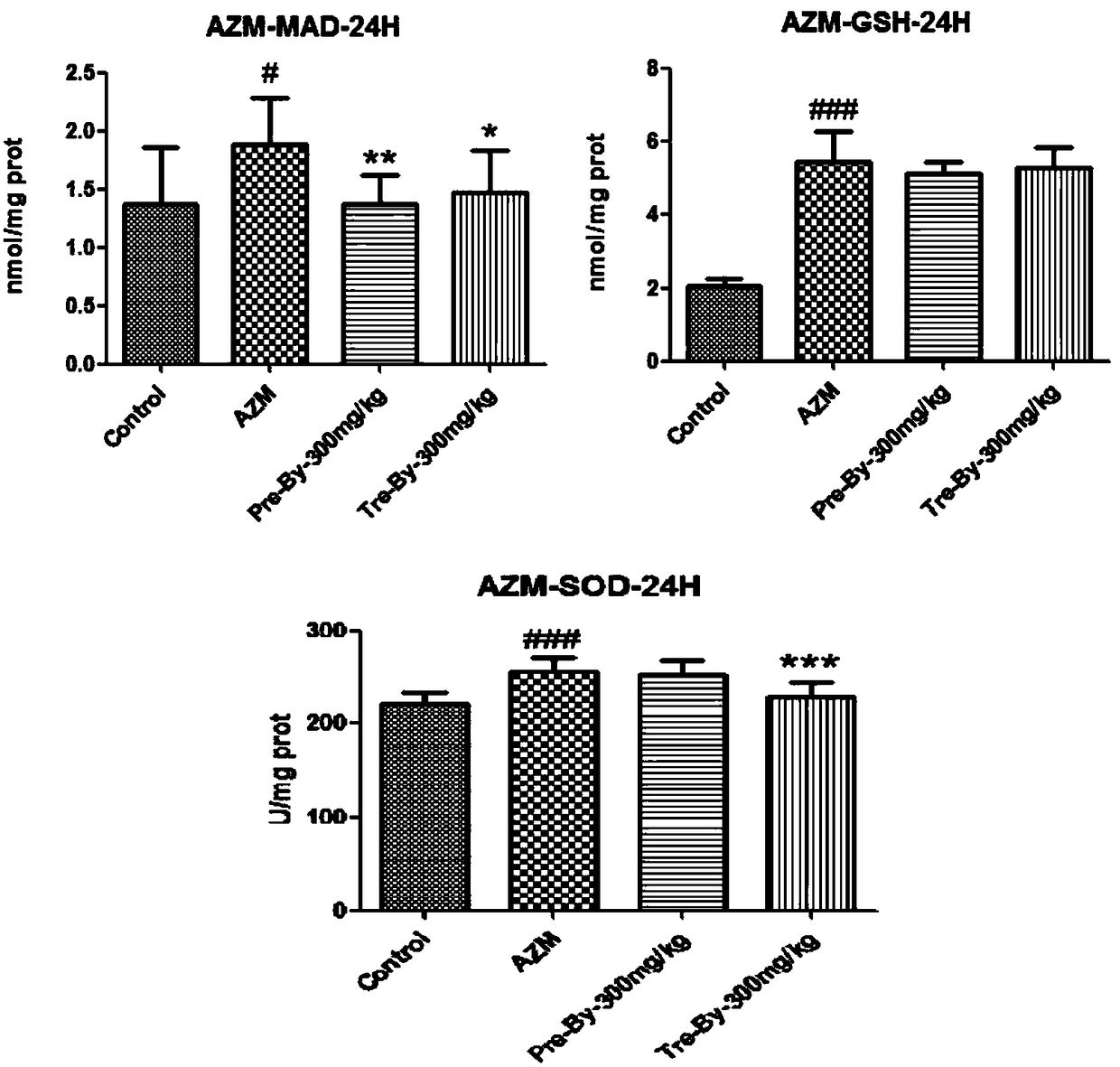
[0044] 1. Protective effect of bicyclol on liver injury induced by azithromycin
[0045] Bicyclol preventive and therapeutic administration groups can reduce the elevation of serum ALT and AST in mice with azithromycin-induced liver injury, which is significantly different from the model control group, and has a certain dose-effect relationship. The results are shown in T...
Embodiment 3
[0145] Embodiment 3: Bicyclol prevention or treatment of drug-induced liver injury caused by diclofenac sodium animal experiment
[0146] 80 healthy male ICR mice (provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., certificate number: SCXK (Beijing) 2012-0001) were randomly divided into 8 groups, namely blank control group, diclofenac sodium liver Injury model control group (model control group), 100mg / kg bicyclol prevention group (low dose prevention group), 200mg / kg bicyclol prevention group (medium dose prevention group), 300mg / kg bicyclol prevention group (high dose prevention group) ), 100mg / kg bicyclol treatment group (low dose treatment group), 200mg / kg bicyclol treatment group (middle dose treatment group), 300mg / kg bicyclol treatment group (high dose treatment group), 10 in every group.
[0147] The mice in the blank control group had a normal diet throughout the test period, and were not given diclofenac sodium and bicyclol, and the mice in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com