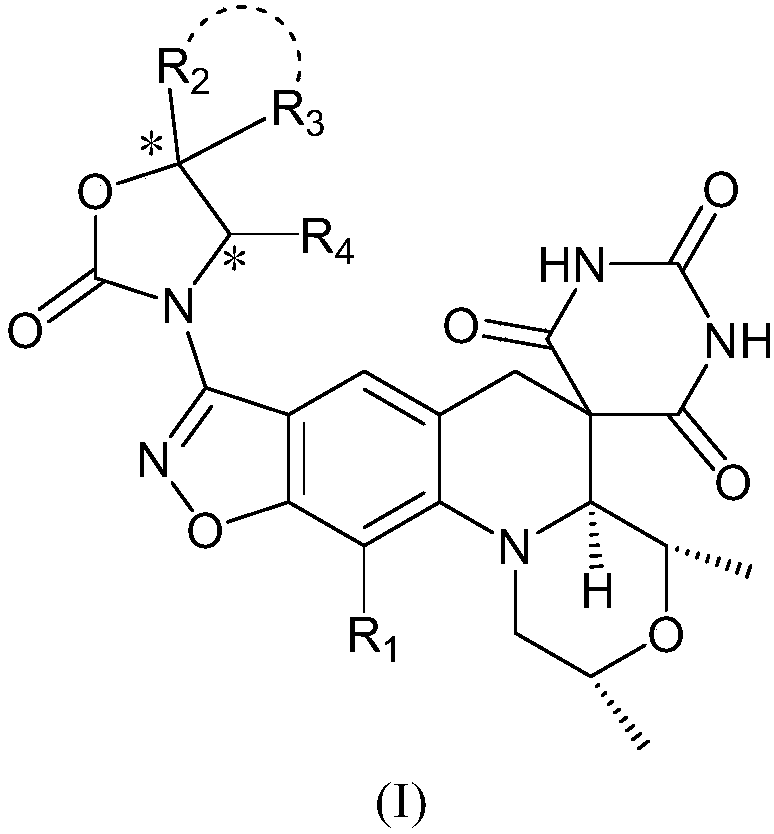
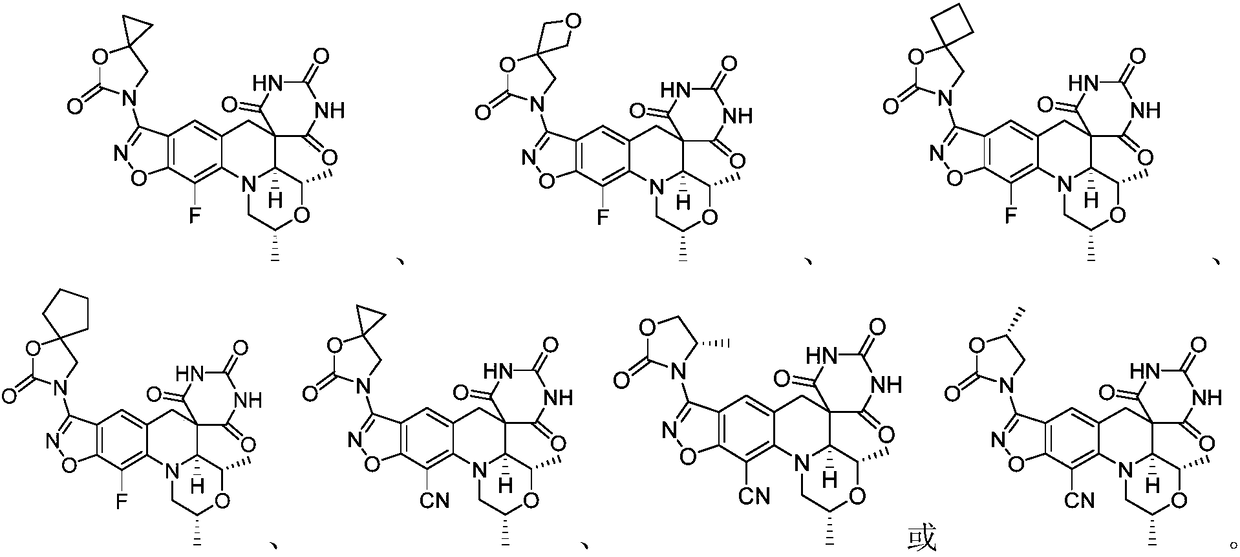
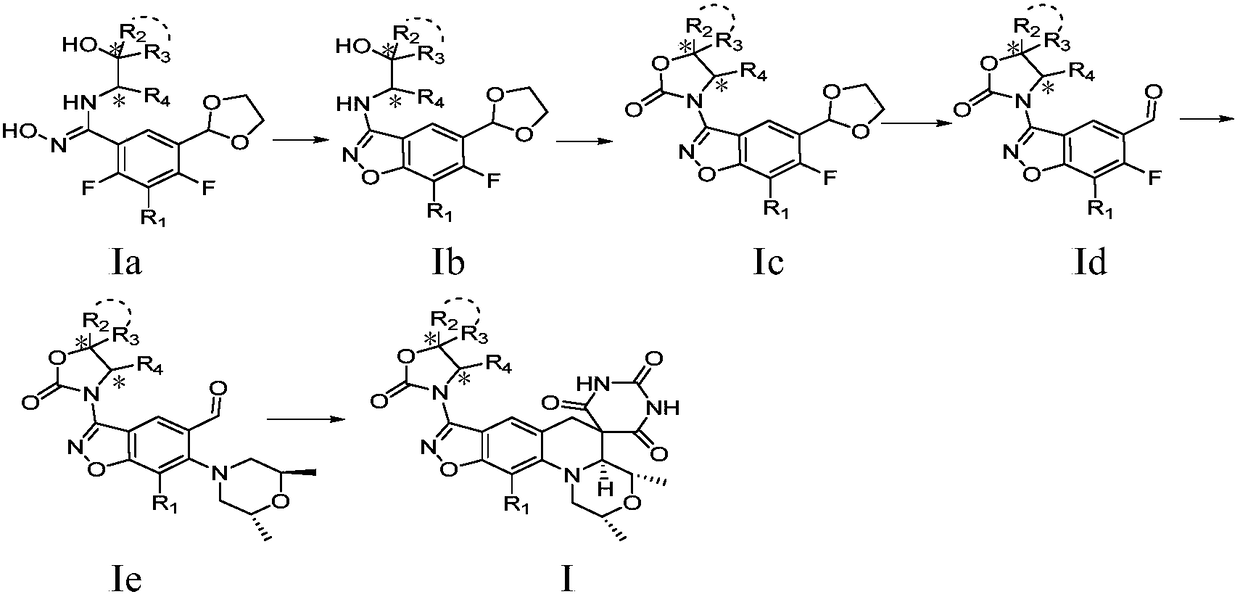
Benzoisoxazole spiropyrimidinetrione compound, preparation method and uses thereof
A compound and mixture technology, applied in organic chemistry methods, organic chemistry, pharmaceutical formulations, etc., can solve the problems of unsatisfactory metabolic properties, limitations, and weak antibacterial activity of spiropyrimidine triketone compounds, etc., and achieve excellent in vivo metabolic properties, good medicinal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1: (2R,4S,4aS)-11-fluoro-2,4-dimethyl-8-(5-oxo-4-oxo-6-azaspiro[2.4]heptane-6-yl )-1,2,4,4a-tetrahydro-2'H,6H-spiro[isoxazol[4,5-g][1,4]oxazine[4,3-a]quinoline-5, 5'-pyrimidine]-2',4',6'(1'H,3'H)-trione (compound 1)
[0111] (a) 1-(((5-(1,3-dioxolan-2-yl)-6,7-difluorobenzo[d]isoxazol-3-yl)amino)methyl)- 1-Cyclopropanol (I-3-1)
[0112]
[0113] Intermediate A (1g, 4.048mmol) was added to 20ml of N,N-dimethylformamide, stirred to dissolve it, N-chlorosuccinimide (650mg, 4.868mmol) was added at room temperature, raised to React at 40°C for 30min, TLC (thin layer chromatography) monitors the completion of the reaction, cool the reaction solution to 0°C, slowly add 1-aminomethyl-1-cyclopropanol (1.06g, 12.144mmol), return to room temperature and react for 1h , TLC monitors that the reaction is complete, adds cesium carbonate (4.567g, 12.144mmol), rises to 60°C for 2h, TLC monitors that the reaction is complete, cools down to room temperature, adds water and et...
Embodiment 2
[0126] Example 2: (2R,4S,4aS)-11-fluoro-2,4-dimethyl-8-(6-oxo-2,5-dioxo-7-azaspiro[3.4]octane- 7-yl)-1,2,4,4a-tetrahydro-2'H,6H-spiro[isoxazol[4,5-g][1,4]oxazin[4,3-a]quinoline -5,5'-pyrimidine]-2',4',6'(1'H,3'H)-trione (compound 2)
[0127] (a) 3-(((5-(1,3-dioxolan-2-yl)-6,7-difluorobenzo[d]isoxazol-3-yl)amino)methyl)oxy Heterobutanol (I-3-2)
[0128]
[0129] According to the synthetic method of intermediate I-3-1, intermediate A (1.2g, 4.857mmol), N-chlorosuccinimide (778mg, 5.828mmol), 3-(aminomethyl)oxetane Butanol (1.25g, 12.142mmol) and cesium carbonate (4.748g, 14.571mmol) were used as raw materials to synthesize 1-(((5-(1,3-dioxolan-2-yl)-6,7-di Fluorobenzo[d]isoxazol-3-yl)amino)methyl)oxetanol 949mg, white solid, yield 59.5%, 1 H NMR (400MHz, DMSO) δ8.08(d, J=5.7Hz, 1H), 7.41(t, J=5.7Hz, 1H), 6.09(s, 1H), 6.01(s, 1H), 4.48(dd , J=15.1, 6.6Hz, 4H), 4.12–3.99(m, 4H), 3.55(d, J=5.8Hz, 2H).
[0130] (b) 7-(5-((1,3-dioxolan-2-yl)-6,7-difluorobenzo[d]isoxazol-3-yl...
Embodiment 3
[0142] Example 3: (2R,4S,4aS)-11-fluoro-2,4-dimethyl-8-(6-oxo-5-oxo-7-azaspiro[3.4]octane-7-yl )-1,2,4,4a-tetrahydro-2'H,6H-spiro[isoxazol[4,5-g][1,4]oxazine[4,3-a]quinoline-5, 5'-pyrimidine]-2',4',6'(1'H,3'H)-trione (compound 3)
[0143] (a) 1-(((5-(1,3-dioxolan-2-yl)-6,7-difluorobenzo[d]isoxazol-3-yl)amino)methyl)cyclo Butanol (I-3-3)
[0144]
[0145] According to the synthetic method of intermediate I-3-1, intermediate A (750mg, 3.036mmol), N-chlorosuccinimide (527mg, 3.947mmol), 1-(aminomethyl)cyclobutanol ( 675mg, 6.679mmol) and cesium carbonate (3.957g, 12.144mmol) as raw materials, synthesis of 1-(((5-(1,3-dioxolan-2-yl)-6,7-difluorobenzo[ d] isoxazol-3-yl)amino)methyl)cyclobutanol 710mg, white solid, yield 72%, 1 H NMR (400MHz, DMSO) δ8.15 (d, J = 5.9Hz, 1H), 7.23 (t, J = 5.4Hz, 1H), 6.08 (s, 1H), 5.27 (s, 1H), 4.11–3.99 (m, 4H), 3.34 (d, J=5.6Hz, 2H), 2.12–1.92 (m, 4H), 1.72–1.45 (m, 2H).
[0146] (b) 7-(5-((1,3-dioxolan-2-yl)-6,7-difluorobenzo[d]isoxazol-3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com