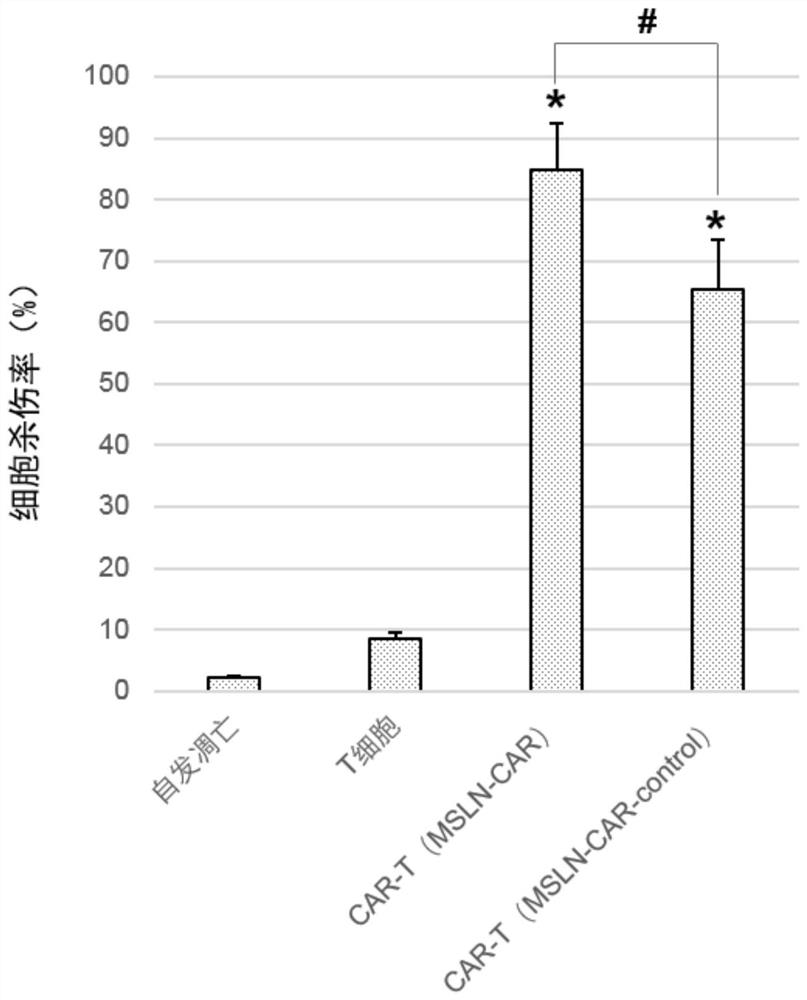
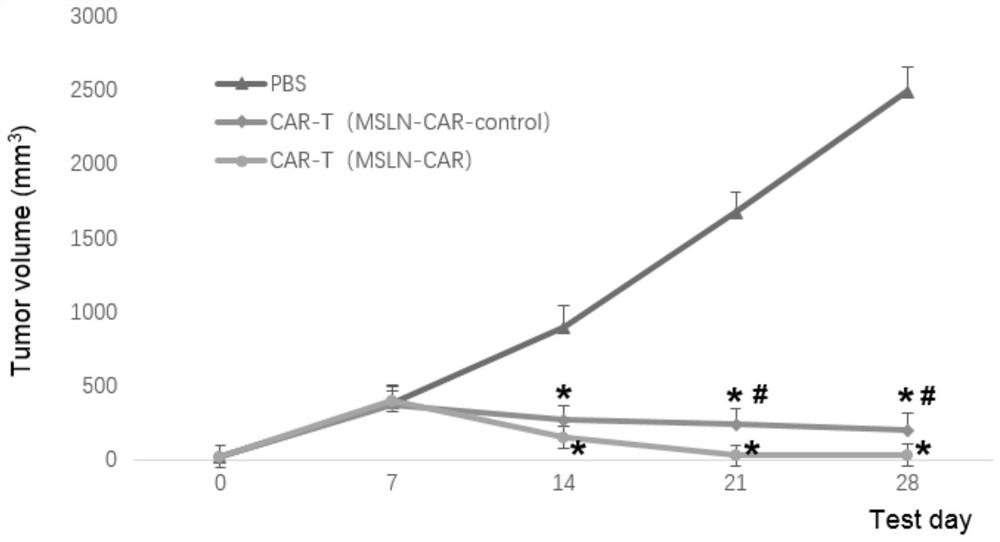
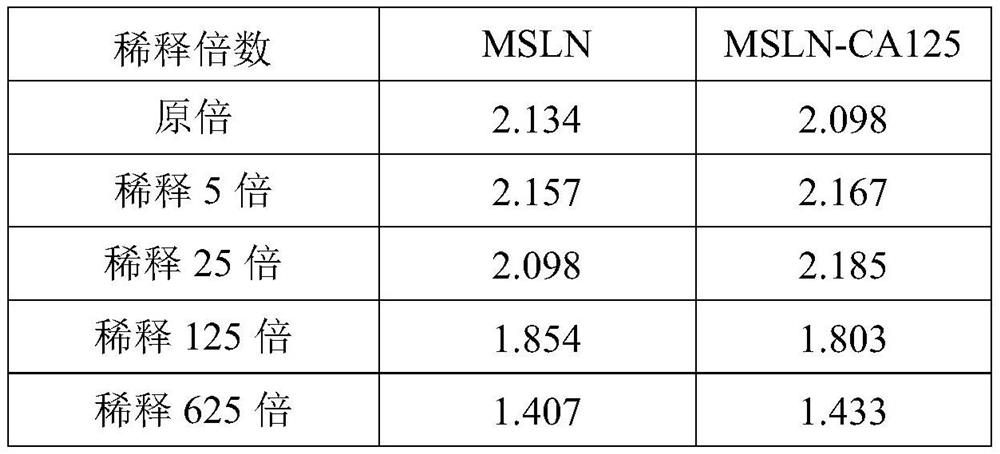
Chimeric antigen receptors targeting msln
A chimeric antigen receptor and antigen technology, applied in the field of biomedicine, to achieve high activity, enhanced therapeutic effect, and small side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of sc-Fv
[0040] 1. Animal immunization and preparation of monoclonal antibodies
[0041] The extracellular domain part of human MSLN protein was used as immunogen, mixed with complete Freund's adjuvant, and immunized 6-week-old female Balb / c mice (the dosage of immunogen was 100 μg / mouse), and the immunization method was subcutaneous multiple Spot immunization; two weeks later, the immunogen was mixed with incomplete Freund's adjuvant, and the mice were boosted (the dosage of immunogen was 50 μg / mouse), and the immunization method was subcutaneous multi-point immunization; every two weeks Booster immunization was carried out in the same way, a total of 3 booster immunizations. On the 7th day after the last booster immunization, the eyeballs of the mice were removed, blood was collected from the orbital venous plexus of the mice, and the serum was separated by centrifugation, and the antibody titer of the serum was detected by ELISA. If P / N≥2.1 ...
Embodiment 2
[0061] Example 2 Construction of Chimeric Antigen Receptor
[0062] Signal peptide, MSLN antigen-binding domain (sc-Fv prepared in Example 1), CD28 transmembrane co-stimulatory domain, 4-1BB transmembrane co-stimulatory domain, CD3ζT cell signaling region, co-expression through whole gene synthesis Runx3 agonist domain:
[0063] MSLN-CAR: signal peptide-MSLN scFv-CD28-4-1BB-CD3ζ-Runx3;
[0064] Wherein the signal peptide sequence is shown in SEQ ID NO: 16;
[0065] The gene sequence number of CD28 is XM_006712862.1; the gene sequence number of 4-1BB is U03397.1; the gene sequence number of CD3ζ is AF228312.1; the sequence selection refers to Sadelain M, Nature biotechnology, 2013,31(1):71 -5 Structures of designed chimeric antigen receptors.
[0066] At the same time, the comparison sequence MSLN-CAR-control is set. Compared with MSLN-CAR, the only difference is that the Runx3 segment is missing.
Embodiment 3
[0067] Example 3 Construction of MSLN-CAR-T lentivirus
[0068] Cloning of MSLN-CAR fragment and MSLN-CAR-control into lentiviral vector pc DNA TM For 3.1(+), the selected insertion site is Nhe I / Bam HI. The sequence was provided by the applicant, and the assembly procedure was completed by Suzhou Gemma Gene Co., Ltd.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com