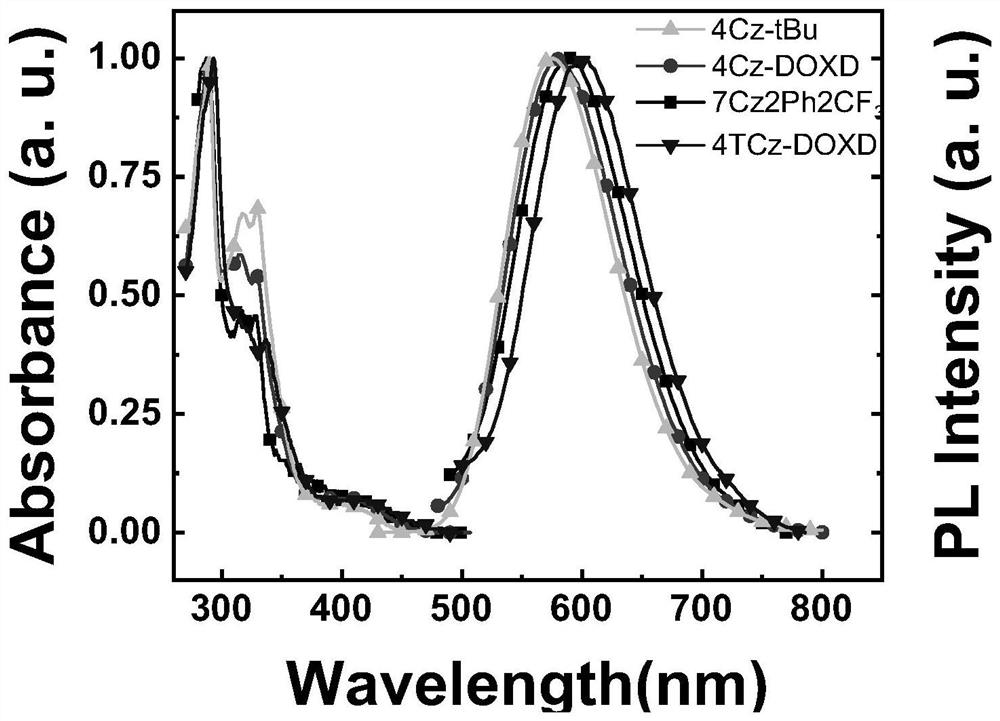
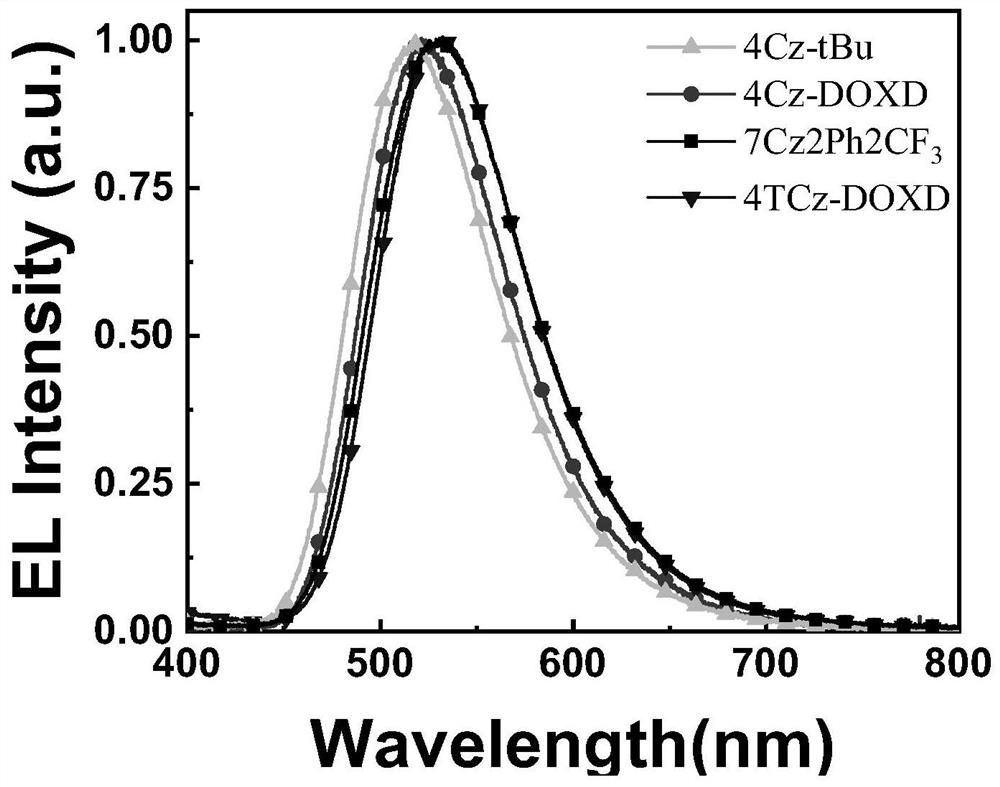
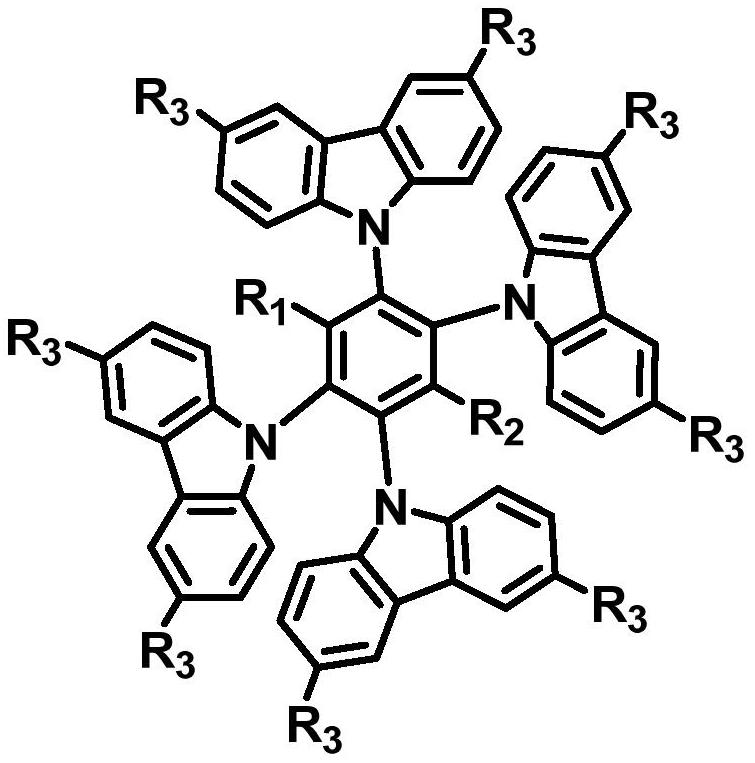
A kind of double-acceptor type multi-substituted carbazole compound with tadf characteristic and its preparation method and application
A compound and multi-substitution technology, applied in chemical instruments and methods, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problems of low fluorescence quantum efficiency and low device efficiency, achieve high electroluminescence efficiency, and easy operation , the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Synthesis of 4Cz-DOXD
[0044] 5,5'-(perfluoro-1,4-phenylene)bis(2-phenyl-1,3,4-oxadiazole) (0.5g, 1.14mmol), carbazole (1.14g, 6.84mmol ), K 2 CO 3(2.84g, 20.53mmol), DMSO 20ml, under the protection of nitrogen, heated at 150°C for 12h. After cooling to room temperature, it was poured into 200 ml of saturated saline and stirred, and a large amount of yellow solid was precipitated, which was filtered by suction and purified by column chromatography to obtain 1 g of a yellow solid product with a yield of 85%. 1H NMR (CDCl 3 ,303k, 500Hz):δ=7.736-7.685(d,8H),7.351-7.315(d,10H),7.160-7.088(t,12H),7.083-7.017(t,8H),6.805-6.754(d, 4H).
[0045]
Embodiment 2
[0046] Embodiment 2: Synthesis of 4TCz-DOXD
[0047] 5,5'-(perfluoro-1,4-phenylene)bis(2-phenyl-1,3,4-oxadiazole) (0.5g, 1.14mmol), 3,6-di-tert-butyl Carbazole (1.91g, 6.84mmol), K 2 CO 3 (2.84g, 20.53mmol), DMSO 20ml, under the protection of nitrogen, heated at 150°C for 12h. After cooling to room temperature, it was poured into 200 ml of saturated saline and stirred, and a large amount of yellow solid was precipitated, which was filtered by suction and purified by column chromatography to obtain 1.45 g of a yellow solid product with a yield of 85%. 1H NMR (CDCl 3 ,303k, 500Hz):δ=7.60(s,8H),7.351-7.315(d,2H),7.182-7.129(d,12H),7.129-7.076(d,8H), 6.988-6.937(d,4H) ,1.31(s,72H).
[0048]
Embodiment 3
[0049] Embodiment 3: Synthesis of 4Cz-tBu
[0050] 5,5'-(perfluoro-1,4-phenylene)bis(2-(4-(tert-butyl)phenyl)-1,3,4-oxadiazole) (0.5g, 0.98mmol) , carbazole (0.911g, 5.45mmol), K 2 CO 3 (2.26g, 16.35mmol), DMSO 20ml, under nitrogen protection, heated at 150°C for 12h. After cooling to room temperature, it was poured into 200 ml of saturated saline and stirred. A large amount of yellow solid was precipitated, which was filtered by suction and purified by column chromatography to obtain 0.96 g of a yellow solid product with a yield of about 85%. 1H NMR (CDCl 3 ,303k, 500Hz):δ=7.742-7.690(s,8H),7.340-7.034(d,8H),7.149-7.086(t,12H),7.086-7.020(t,8H),6.738-6.685(d, 4H), 1.27(s, 18H).
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com