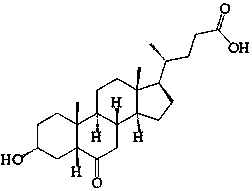
3alpha-hydroxyl-5alpha-cholanic acid synthesis method
A synthetic method and technology of cholanic acid, applied in the direction of steroids, organic chemistry, etc., to achieve the effect of cheap starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
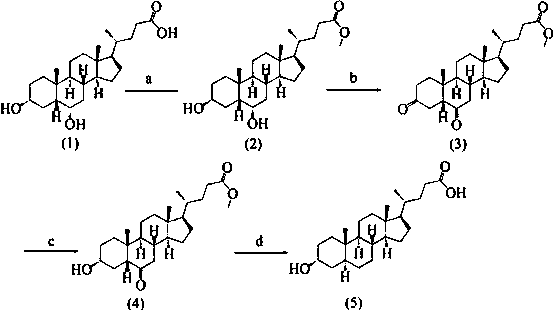
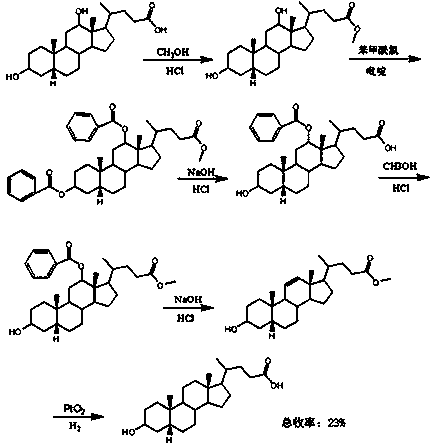
[0038] One, the synthesis of formula (2) compound
[0039] Take 10 g of hyodeoxycholic acid (25.5 mmol), add 100 mL of methanol, then cool down to 5 °C, add 0.5 mL of concentrated sulfuric acid dropwise within about 10 min, stir until the solid dissolves, raise to 25 °C, and stir for 8 h , HPLC detection showed that the reaction was complete, then adding 100 mL of saturated sodium bicarbonate solution to quench the reaction, concentrating under reduced pressure, extracting with 150 mL of ethyl acetate, combining the organic phases, followed by 2*150 mL of saturated sodium bicarbonate, 3*100 Wash with mL of saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 10.4 g of a white solid with a molar yield of 100%. 1 H-NMR (400 MHz, CDCl 3 / TMS): δ = 0.66 (3 H, s, 18-H), 0.94 (6 H, t, J = 1.4 Hz), 3.63 (1 H, m, 6α-H), 3.69 (3 H, s) , 4.07(1H, m, 3α-H).
[0040] Two, the synthesis of formula (3) compound
[0041] Take 10 g o...
Embodiment 2
[0048] One, the synthesis of formula (2) compound
[0049] Take 10 g of hyodeoxycholic acid, add 100 mL of methanol, then cool down to 0°C, add 0.5 mL of concentrated sulfuric acid drop by drop within about 10 min, stir until the solid dissolves, rise to 25°C, stir for 12 h, and detect the reaction by HPLC complete, then add 100 mL of saturated sodium bicarbonate solution to quench the reaction, concentrate under reduced pressure, extract with 150 mL of ethyl acetate, and wash the organic phase with 2*150 mL of saturated sodium bicarbonate and 3*100 mL of saturated brine, respectively. Dry over sodium sulfate, filter, and concentrate to obtain 10.0 g of white solid, with a molar yield of 96.6%.
[0050] Two, the synthesis of formula (3) compound
[0051] Take 10 g of the compound of formula (2), add 200 mL of dichloromethane, stir at room temperature to dissolve, add 16 g of oxidant PCC, react for 20 min after the dropwise addition, TLC detection shows that the reaction is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com