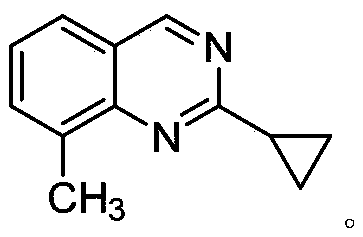
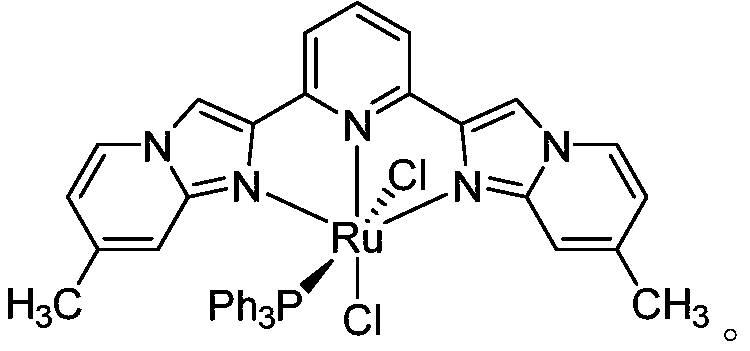
The synthetic method of 2-cyclopropyl-8-methylquinazoline
A technology of methylquinazoline and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of environmental friendliness and atom economy, and achieve the effects of shortening reaction time, high reaction efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 15ml high-pressure tube, add 51.41mg (0.375mmol) (2-amino-3-methylphenyl) methanol, 16.76mg (0.25mmol) cyclopropylcarbonitrile, 5.1mg (0.3equiv) sodium ethoxide, 1mL tert Amyl alcohol was stirred magnetically under an air atmosphere, and reacted at 130° C. for 2 h. After TLC analysis, No Reaction.
Embodiment 2
[0021] The synthetic method of the 2-cyclopropyl-8-methylquinazoline of the present embodiment, the steps are as follows:
[0022] In a 15ml high-pressure tube, add 51.41mg (0.375mmol) (2-amino-3-methylphenyl) methanol, 16.76mg (0.25mmol) cyclopropylcarbonitrile, 1.93mg (1mol%) pincer metal ruthenium ( II) Compound, 5.1 mg (0.3 equiv) sodium ethoxide, 1 mL tert-amyl alcohol, magnetically stirred under air atmosphere, reacted at 130° C. for 2 h. According to TLC analysis, the raw material cyclopropylcarbonitrile had reacted completely. Vacuum rotary evaporation, separation and purification by thin layer chromatography, the quality of the product 2-cyclopropyl-8-methylquinazoline was 20.6 mg, and the yield was 45%. product by 1 H NMR, 13 Confirmed by C NMR. 1 H NMR (600MHz, CDCl3) δ9.18 (s, 1H), 7.65 (d, J = 7.5Hz, 2H), 7.39 (t, J = 7.5Hz, 1H), 2.71 (s, 3H), 2.47–2.38 (m,1H),1.30–1.23(m,2H),1.15–1.09(m,2H).13C NMR(101MHz,CDCl3)δ167.4,160.2,149.5,135.9,133.6,125.8,124.8,123....
Embodiment 3
[0024] The synthetic method of the 2-cyclopropyl-8-methylquinazoline of the present embodiment, the steps are as follows:
[0025] In a 15ml high-pressure tube, 34.27mg (0.25mmol) (2-amino-3-methylphenyl) methanol, 16.76mg (0.25mmol) cyclopropylcarbonitrile, 1.93mg (1mol%) pincer metal ruthenium (II ) compound, 6.0 mg (0.6 equiv) of sodium hydroxide, 1 mL of 1,4-dioxane, magnetically stirred in an air atmosphere, and reacted at 130° C. for 2 h. According to TLC analysis, the raw material cyclopropylcarbonitrile had reacted completely. Vacuum rotary evaporation, separation and purification by thin layer chromatography, the product 2-cyclopropyl-8-methylquinazoline was 30.2 mg, and the yield was 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com