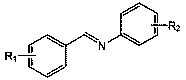
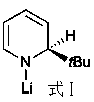
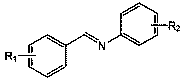
Applications of anilino lithium in catalytic hydroboration reaction of imine and borane
A technology of lithium anilide and catalytic imine, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of low yield and long reaction time of the catalytic system , the catalyst is expensive and other problems, to achieve the effect of good universality, short reaction time, simple and controllable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Lithium anilide catalyzes the hydroboration reaction of benzidine and pinacol borane
[0022] In the reaction flask that has been dehydrated and deoxygenated, under the protection of argon, add 0.5 mmol of benzylaniline, add 100 ulTHF, then add 0.5 mmol (0.0726 mL) borane with a pipette and mix well, and finally add 43.4 ul of lithium anilide Tetrahydrofuran solution (0.5759M) (5 mol% dosage, the same below), after reacting for 2 h, draw a drop into the NMR tube with a dropper, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 91%. NMR data of the product: 1 H NMR (CDCl 3 , 400 MHz)δ: 7.29~7.12(m, 9H), 6.88~6.84 (t, 1H), 4.69 (s, 2H), 1.29 (s, 12H).
Embodiment 2
[0023] Embodiment 2: Lithium anilide catalyzes the hydroboration reaction of benzidine and pinacol borane
[0024] In the reaction flask that has been dehydrated and deoxygenated, add 0.5 mmol of benidine aniline under the protection of argon, add 100 ulTHF, then add 0.55 mmol (0.0798 mL) borane with a pipette and mix well, and finally add 43.4 ul lithium anilide Tetrahydrofuran solution (0.5759M) (5 mol% dosage, the same below), after reacting for 2 h, draw a drop into the NMR tube with a dropper, and add CDCl3 to form a solution. The calculated 1H spectrum yield was 95%. NMR data of the product: 1H NMR (CDCl3, 400 MHz)δ: 7.29~7.12(m, 9H), 6.88~6.84 (t, 1H), 4.69 (s, 2H), 1.29 (s, 12H).
Embodiment 3
[0025] Embodiment 3: Lithium anilide catalyzes the hydroboration reaction of benzidine and pinacol borane
[0026] In the reaction flask that has been dehydrated and deoxygenated, add 0.5 mmol of benzidine aniline under the protection of argon, add 100 ulTHF, then add 0.6 mmol (0.0871 mL) borane with a pipette and mix well, and finally add 43.4 ul lithium anilide Tetrahydrofuran solution (0.5759M) (5 mol% dosage), after reacting for 2 h, draw a drop into the nuclear magnetic tube with a dropper, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (CDCl 3 , 400 MHz) δ :7.29~7.12(m, 9H), 6.88~6.84 (t, 1H), 4.69 (s, 2H), 1.29 (s, 12H).
[0027] Replacing the lithium anilide with the lithium amidide compound of formula I did not yield the product.
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com