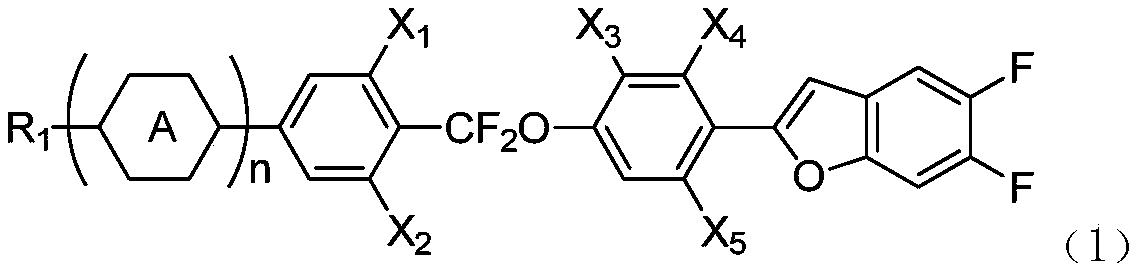
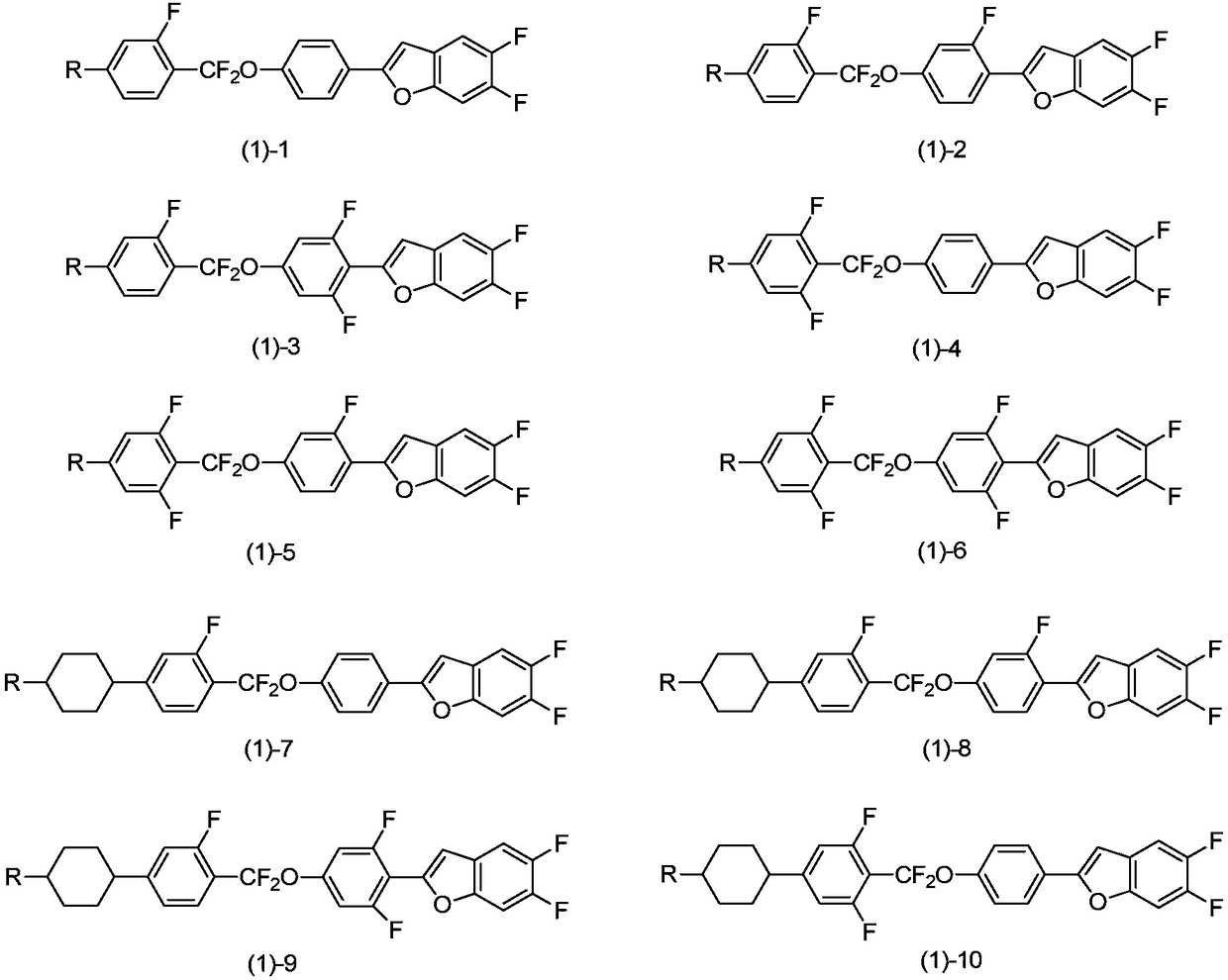
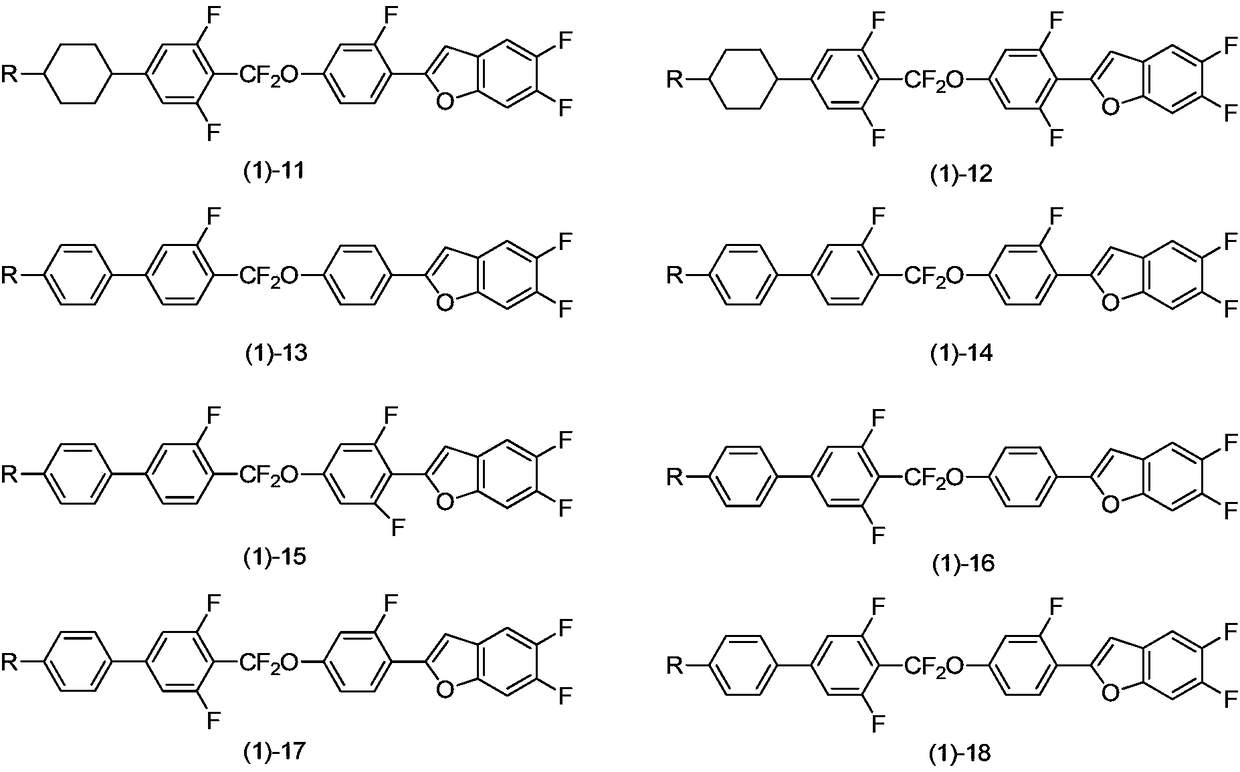
Benzofuran liquid crystal compound having difluoromethyl ether bridge, and composition thereof
A liquid crystal compound, benzofuran technology, applied in the field of liquid crystal display, benzofuran liquid crystal compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] 2-(4-((3,5-difluoro-4'-propyl-4-biphenyl)difluoromethoxy)-2,6-difluorophenyl)-5,6-difluorobenzene Synthesis of furans
[0120] The specific structure is as follows:
[0121]
[0122] The preparation process is as follows:
[0123] (1) Synthesis of intermediate 4‐(difluoromethyl bromide)‐3,5‐difluoro‐4'‐propyl‐1,1'‐biphenyl
[0124]
[0125] Under the protection of nitrogen, add 3,5-difluoro-4'-propyl-1,1' to a 250mL three-neck round bottom flask equipped with mechanical stirring, thermometer, constant pressure dropping funnel, reflux condenser, and nitrogen air tube. - biphenyl (34.8g, 0.15mol), dry THF (250mL), liquid nitrogen cooled to -78 ° C, began to drop n-butyllithium (79.1mL, 0.19mol, 2.4M n-hexane solution), dropwise After the incubation reaction for 1 hour, add the pre-frozen THF (50mL) solution dissolved in difluorodibromomethane (63g, 0.3mol) dropwise, continue the incubation reaction for 1.5 hours after the drop, gradually rise to room temperature ...
Embodiment 2
[0151] Synthesis of 2-(4-((2,6-difluoro-4-propylphenyl)difluoromethoxy)-2,6-difluorophenyl)-5,6-difluorobenzofuran
[0152] The specific structure is as follows:
[0153]
[0154] The preparation process is as follows:
[0155] (1) Synthesis of intermediate 2-(difluorobromomethyl)-1,3-difluoro-5-propylbenzene
[0156]
[0157] Using 1,3-difluoro-5-propylbenzene to replace the intermediate 4-(difluoromethyl bromide)-3,5-difluoro-4'-propyl-1,1'-biphenyl in Example 1 3,5-difluoro-4'-propyl-1,1'-biphenyl in the step, the compound 2-(difluorobromomethyl)-1,3-difluoro-5- Propylbenzene.
[0158] (2) Synthesis of intermediate 4-(5,6-difluorobenzofuran-2-yl)-3,5-difluorophenol
[0159] Experiment with embodiment 1.
[0160] (3) The target compound 2-(4-((2,6-difluoro-4-propylphenyl)difluoromethoxy)-2,6-difluorophenyl)-5,6-difluorobenzene Synthesis of furans
[0161]
[0162] Using 2-(difluorobromomethyl)-1,3-difluoro-5-propylbenzene instead of Example 1 compound 2-(4-((...
Embodiment 3
[0167] 2-(4-((2,6-difluoro-4-(4-propylcyclohexyl)phenyl)difluoromethoxy)-2,6-difluorophenyl)-5,6-difluoro Synthesis of Benzofuran
[0168] The specific structure is as follows:
[0169]
[0170] The preparation process is as follows:
[0171] (1) Synthesis of intermediate 2-(difluorobromomethyl)-1,3-difluoro-5-(4-propylcyclohexyl)benzene
[0172]
[0173] Using 1,3-difluoro-5-(4-propylcyclohexyl)benzene to replace the intermediate 4-(difluoromethyl bromide)-3,5-difluoro-4'-propyl-1 in Example 1, 3,5-difluoro-4'-propyl-1,1'-biphenyl in the 1'-biphenyl synthesis step, the compound 2-(difluorobromomethyl)-1,3 -Difluoro-5-(4-propylcyclohexyl)benzene.
[0174] (2) Synthesis of intermediate 4-(5,6-difluorobenzofuran-2-yl)-3,5-difluorophenol
[0175] Experiment with embodiment 1.
[0176] (3) The target compound 2-(4-((2,6-difluoro-4-(4-propylcyclohexyl)phenyl)difluoromethoxy)-2,6-difluorophenyl)-5 , Synthesis of 6-Difluorobenzofuran
[0177]
[0178] Using 2-(difluoro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com