Preparation method and application of rhabdovirus expressed swine fever E2 subunit vaccine
A subunit vaccine and baculovirus technology, which is applied in the field of preparation of swine fever E2 subunit vaccine, can solve the problems of not being able to prevent and control swine fever, and can not distinguish between infected and immunized animals, and achieve good safety and immunogenicity Strong, high-yielding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The expression of embodiment 1 hog fever SP-E2 protein
[0050] 1) Amplification of the target gene
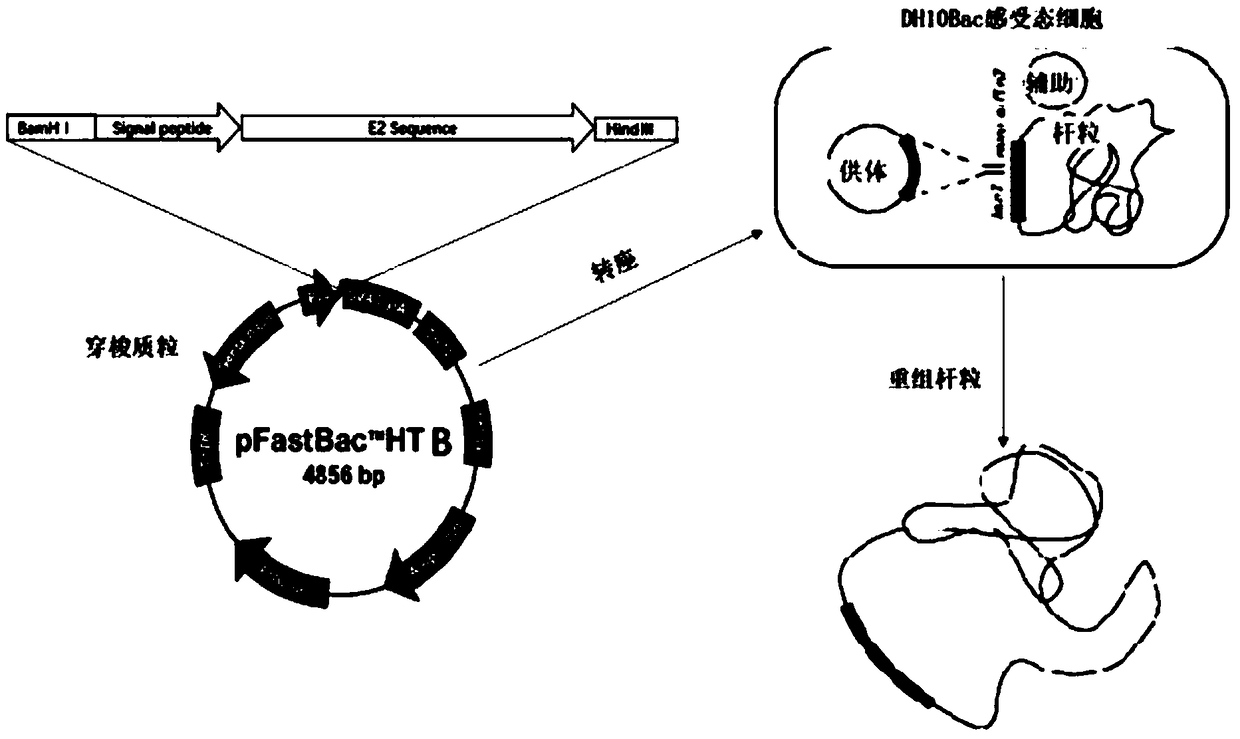
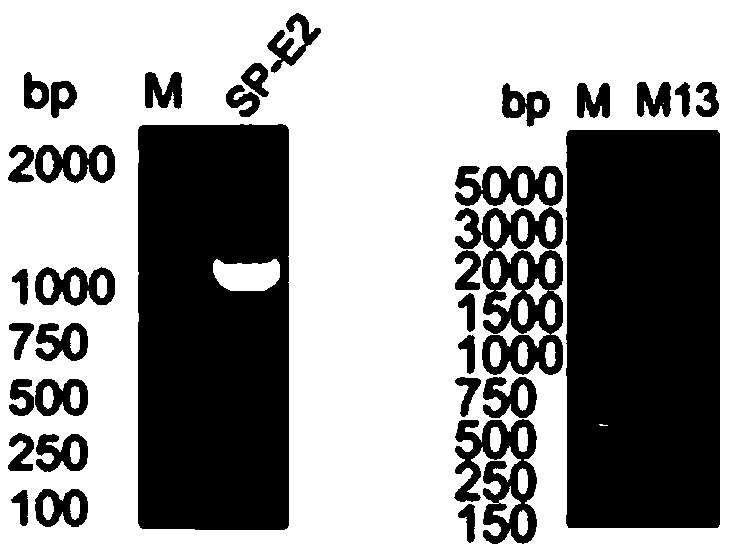
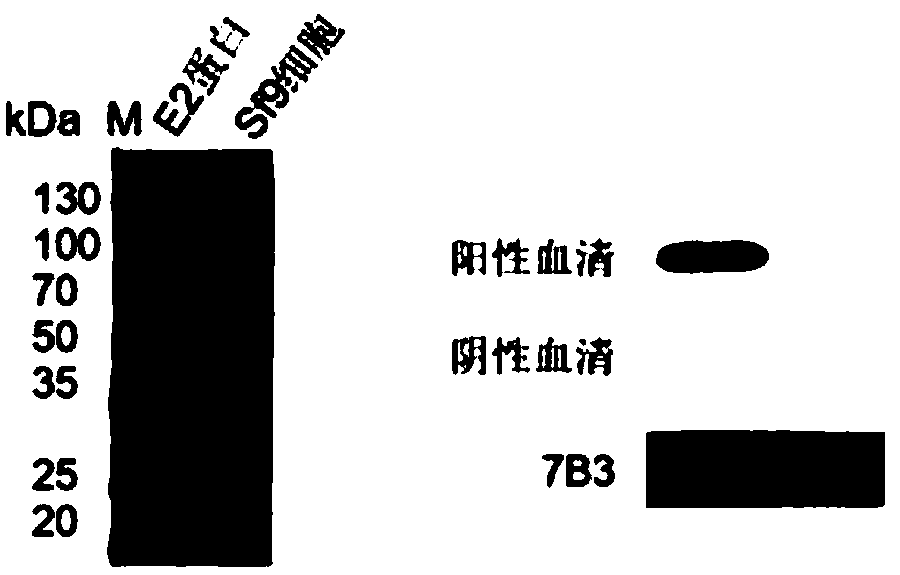
[0051] Primers SP-F, SP-R, E2-F, E2-R were designed according to Genbank, SP signal peptide was amplified by SP-F, SP-R first, and E2 fragment was obtained by amplifying E2-F, E2-R. With SP, E2 as template again, SP-F, E2-R primer amplification obtains target gene SP-E2 (such as figure 2 shown).
[0052] 2) Construction of the shuttle plasmid
[0053] The pFastBacHTB plasmid was digested with EcoRI and XholⅠ restriction endonucleases at 37°C for 2 hours and then recovered. The digested vector and the target gene were recombined with homologous recombination at 37°C for 30 minutes, transformed into Ecoli competent cells, identified by spot picking, and positive bacteria were sent sequencing.
[0054] 3) Acquisition of recombinant bacmid
[0055] Transform the positive plasmid into DH10Bac competent cells, pick white spots after 48h, and carry out PCR identification w...
Embodiment 2
[0066] Example 2 Preparation and animal immunization experiments of the CSF SP-E2 protein subunit vaccine of the present invention.
[0067] The SP-E2 protein expressed in Example 1 was mixed with Seppic 206 water adjuvant at a ratio of 1:1 at 350 rpm for 10 min, sealed and stored at 4°C in the dark.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com