Methods of heat inactivation of adenovirus
A technology of adenovirus and virus, which is applied in the field of thermal inactivation of adenovirus, can solve the problems of AAV vector genome damage or degradation, reducing the quality and quantity of AAV vector, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Preparation of AAV Vectors
[0038] AAV vectors can be prepared in mammalian or insect cells by a variety of methods known in the art. Any production method will be suitable for producing the starting material of the present invention as long as the result of the production method is a sample containing AAV and helper virus. The manufacturing process involves providing cells with a plasmid containing the AAV vector genome, with AAV rep and cap gene functions, and additional helper functions. Other helper functions can be provided, for example, by AV infection, by plasmids carrying all necessary AV helper function genes, or by other viruses such as HSV or baculovirus. AAV production methods suitable for use in the methods of the present invention include Clark et al., Human Gene Therapy 6: 1329-1341 (1995); Martin et al., Human Gene Therapy 24: 253-269 (2013); Thorne et al., Human Gene Therapy 20: 707 -714 (2009); Fraser Wright, Human Gene Therapy 20:698-706 (2009); Vi...
Embodiment 1
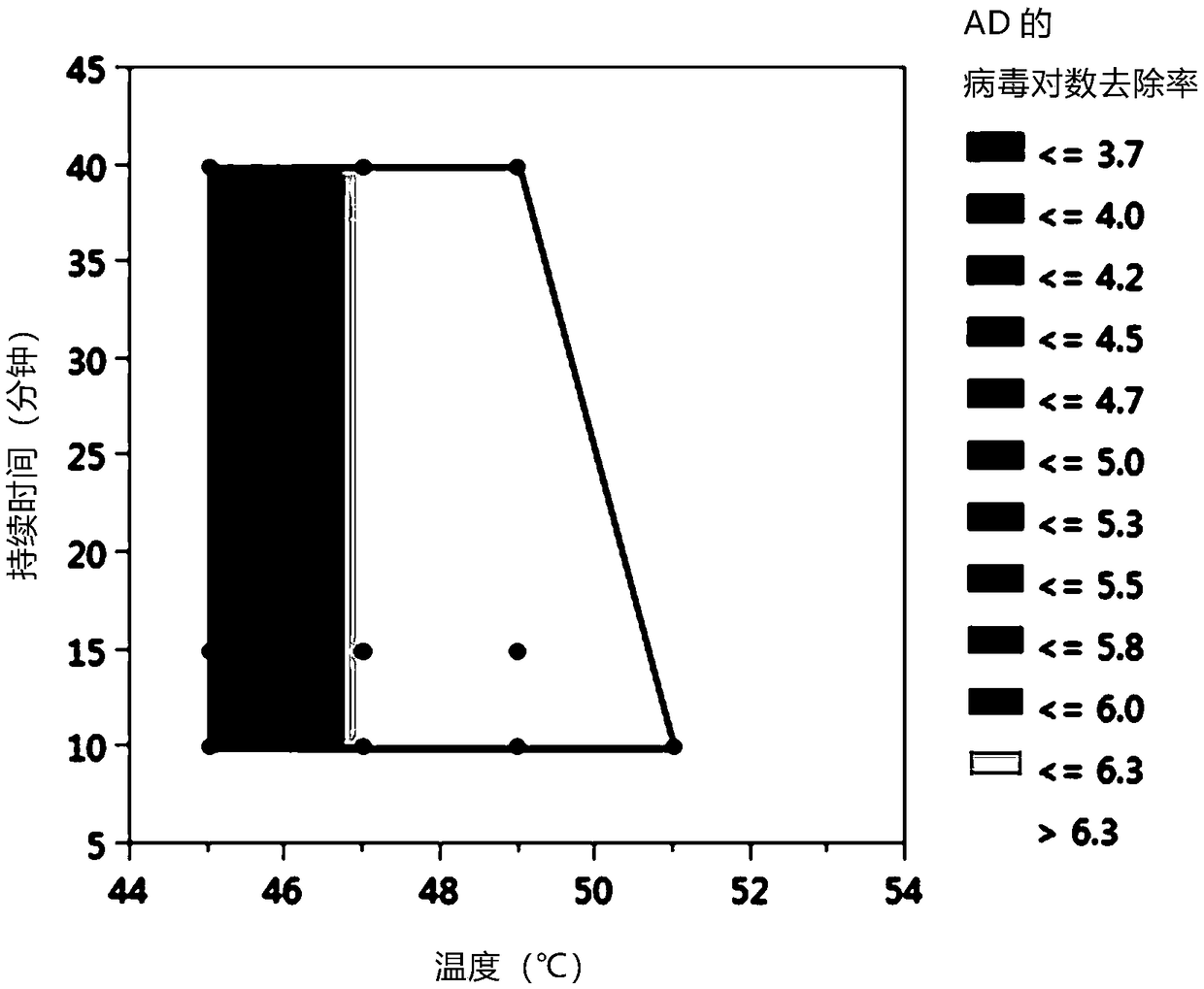
[0062]Ad5AV particles were produced using standard techniques and minimally purified by CsCl gradients. Ad5 particles were dialyzed into background buffer (40 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane, 20 mM HEPES, 20 mM citrate, 200 mM NaCl, 0.001% w / v) Pluronic F68 , pH 8.0). Heat using a thermal cycler to immediately heat the sample to the desired temperature, hold it at the desired temperature, and then immediately cool the sample. Ad5 particles in buffer were contained in polypropylene tubes in volumes below 300 μL. Ad5AV samples were heated to 45°C, 47°C, 49°C or 51°C for 10 minutes, 15 minutes or 40 minutes, except for Ad5AV samples heated to 51°C, which were held at that temperature for 10 minutes only. After heat treatment, use QuickTiter according to manufacturer's instructions TM Ad5 titration immunoassay kit (Cell Biology Laboratories, San Diego, CA) measures Ad5 infectivity.
[0063] figure 1 Heat inactivation data for Ad5AV is shown, shown as Log Rem...
Embodiment 2
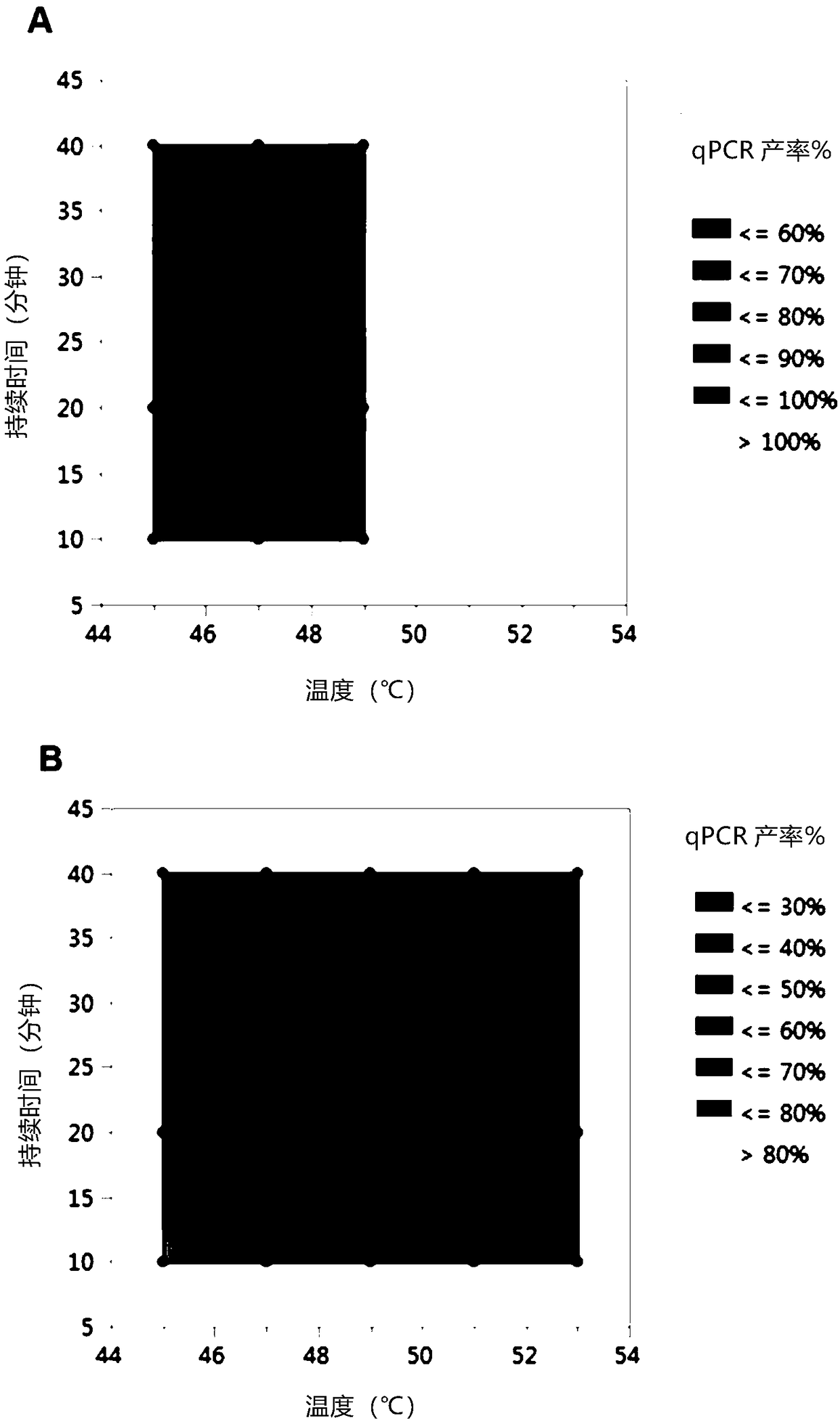
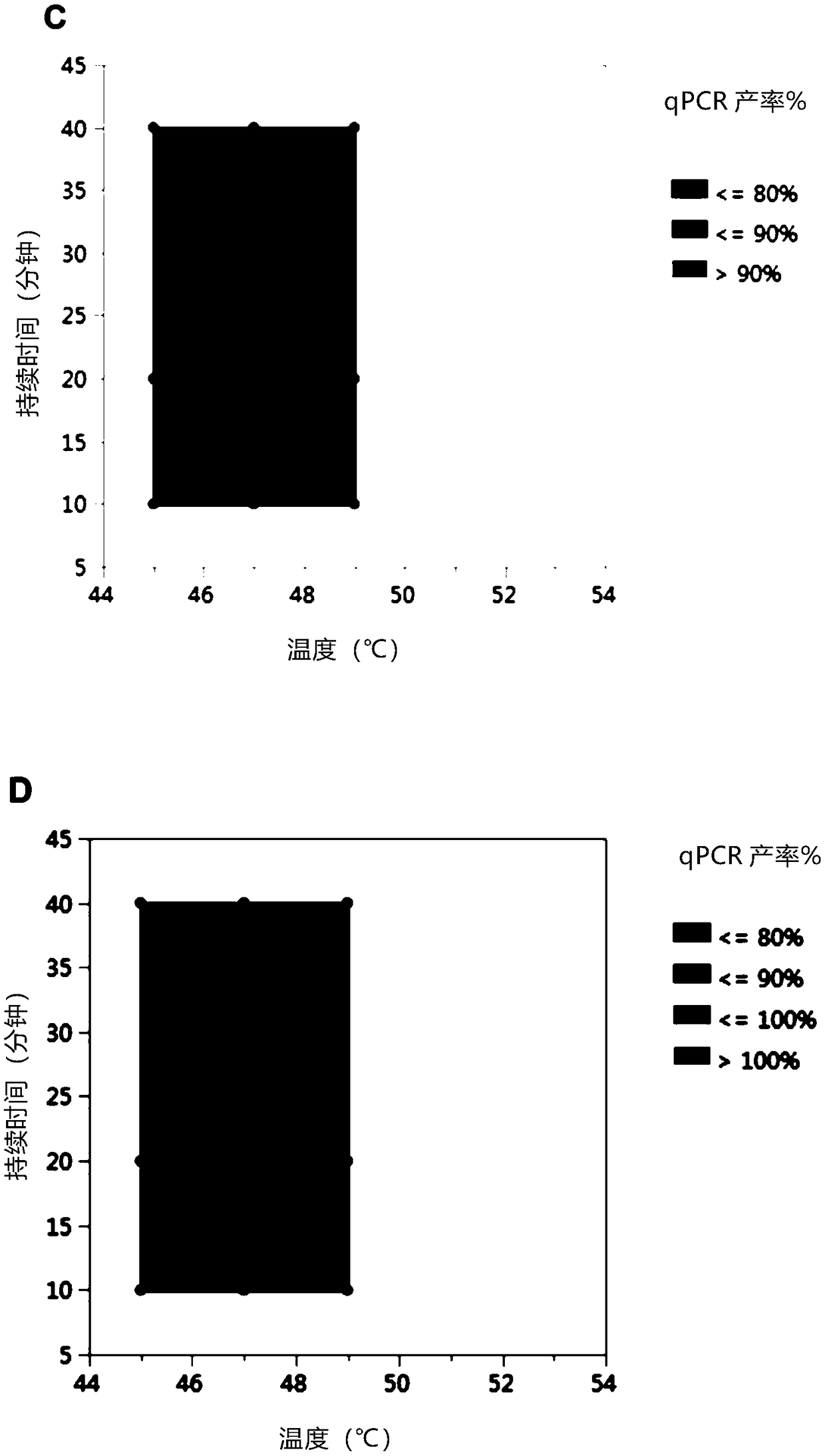
[0065] Detect different serotypes and AAV vectors containing different genome types and sizes, and test the effect of AV heat inactivation protocols on the stability of AAV genomes. Two serotypes, AAV8 and hu37, and three different genomes were tested. The following are the detection of AAV vectors:
[0066] (A) hu37 capsid containing single-chain construct (4.7kb)
[0067] (B) hu37 capsid (5.1 kb) containing the oversized single-chain construct
[0068] (C) AAV8 capsid containing a small single-chain construct (4.1kb)
[0069] (D) AAV8 capsid containing self-complementary construct (4.6kb)
[0070] AAV vectors (A) (C) and (D) were prepared in HEK293 cells and particles (B) were prepared in HeLa cells. All supports were minimally purified by affinity chromatography or iodine gradients using standard techniques. Vehicle was dialyzed into background buffer (40 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane, 20 mM HEPES, 20 mM citrate, 200 mM NaCl, 0.001% (w / v) Pluronic F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com