Crystal form of larotretinib bisulfate and its preparation and application
A bisulfate and crystal form technology, which is applied in the field of larotretinib bisulfate crystal form and its preparation and application, can solve the problems of low solubility of anhydrous crystal form, affecting therapeutic effect, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0135] (3) The preparation method of the crystal form has the characteristics of simple operation, low cost, and is suitable for application in drug research and development and industrial production;
[0136] (4) The pharmaceutical composition prepared with the crystal form has better dissolution rate, and has excellent absorption performance and bioavailability after being administered to patients.
Embodiment 1
[0148] Embodiment 1: Preparation of crystal form AZT-I
[0149] Weigh 47.1 mg of the bisulfate salt of the compound of formula (I), dissolve it in 0.5 mL of N-methylpyrrolidone, and filter. At 25°C, 0.2 mL of the filtrate was slowly dropped into 2.0 mL of chloroform, and stirred until solids were precipitated. The obtained solid is the hydrogen sulfate salt crystal form AZT-I of the compound of formula (I).
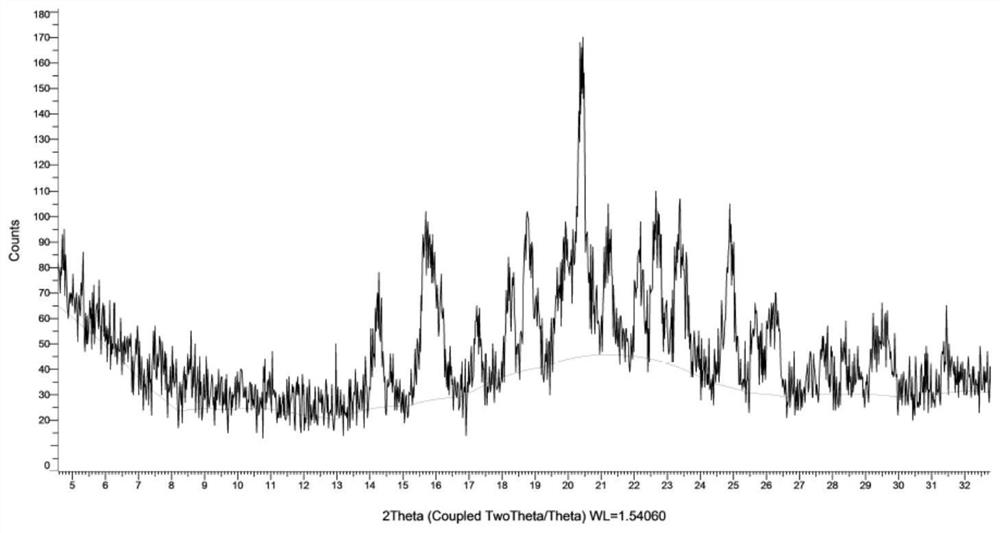
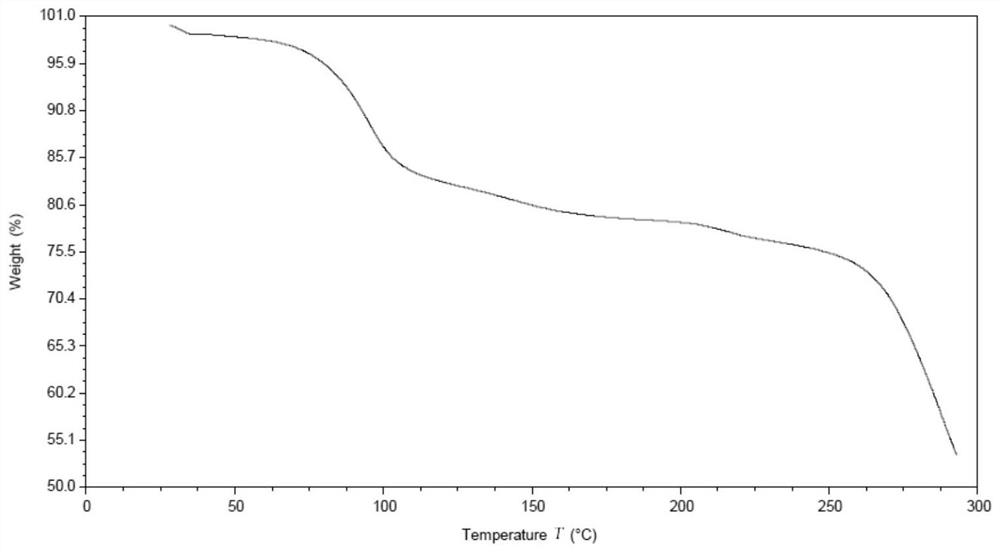
[0150] Carry out XRPD test to the obtained solid, its X-ray powder diffraction pattern is as follows figure 1 Shown; Carry out TGA test to gained solid, its spectrogram is as figure 2 shown.
[0151] From figure 1 It can be seen that the main diffraction peaks and relative intensities of crystal form AZT-I are shown in Table 1.
[0152] Table 1
[0153]
[0154]
[0155] From figure 2 It can be seen that the weight loss of crystal form AZT-I is about 18% at 25-190°C.
Embodiment 2
[0156] Embodiment 2: Preparation of crystal form AZT-I
[0157] Weigh 30.0mg of hydrogen sulfate compound of formula (I), dissolve in 0.2mL N,N-dimethylacetamide, and filter. At 25°C, the filtrate was placed in a closed atmosphere of chloroform until solids were precipitated.
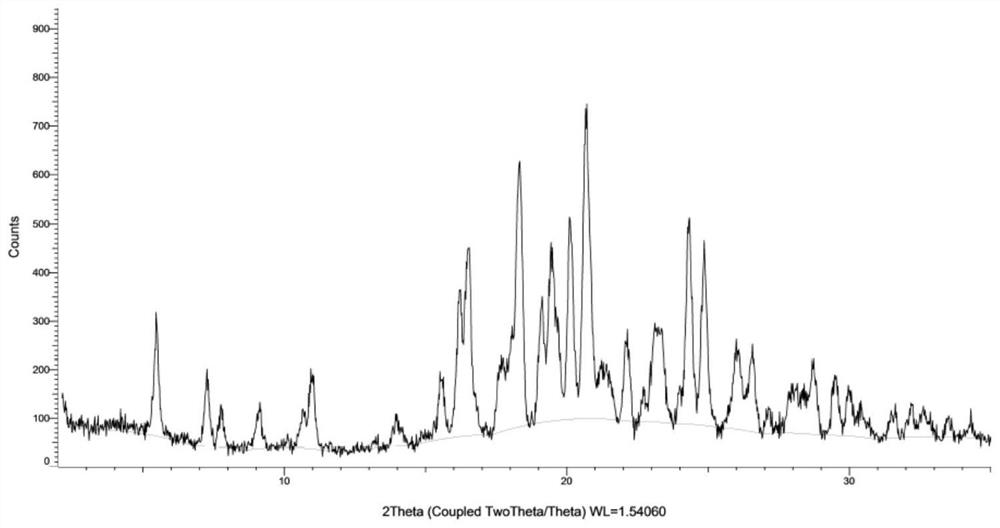
[0158] Carry out XRPD test to the obtained solid, its X-ray powder diffraction pattern is basically as figure 1 As shown, the obtained solid is the hydrogen sulfate salt crystal form AZT-I of the compound of formula (I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com