Method for preparing 16a-hydroxyl prednisolone product
A technology of hydroxyprednisolone and prednisolone dehydroxyacetate, applied in the direction of steroids, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
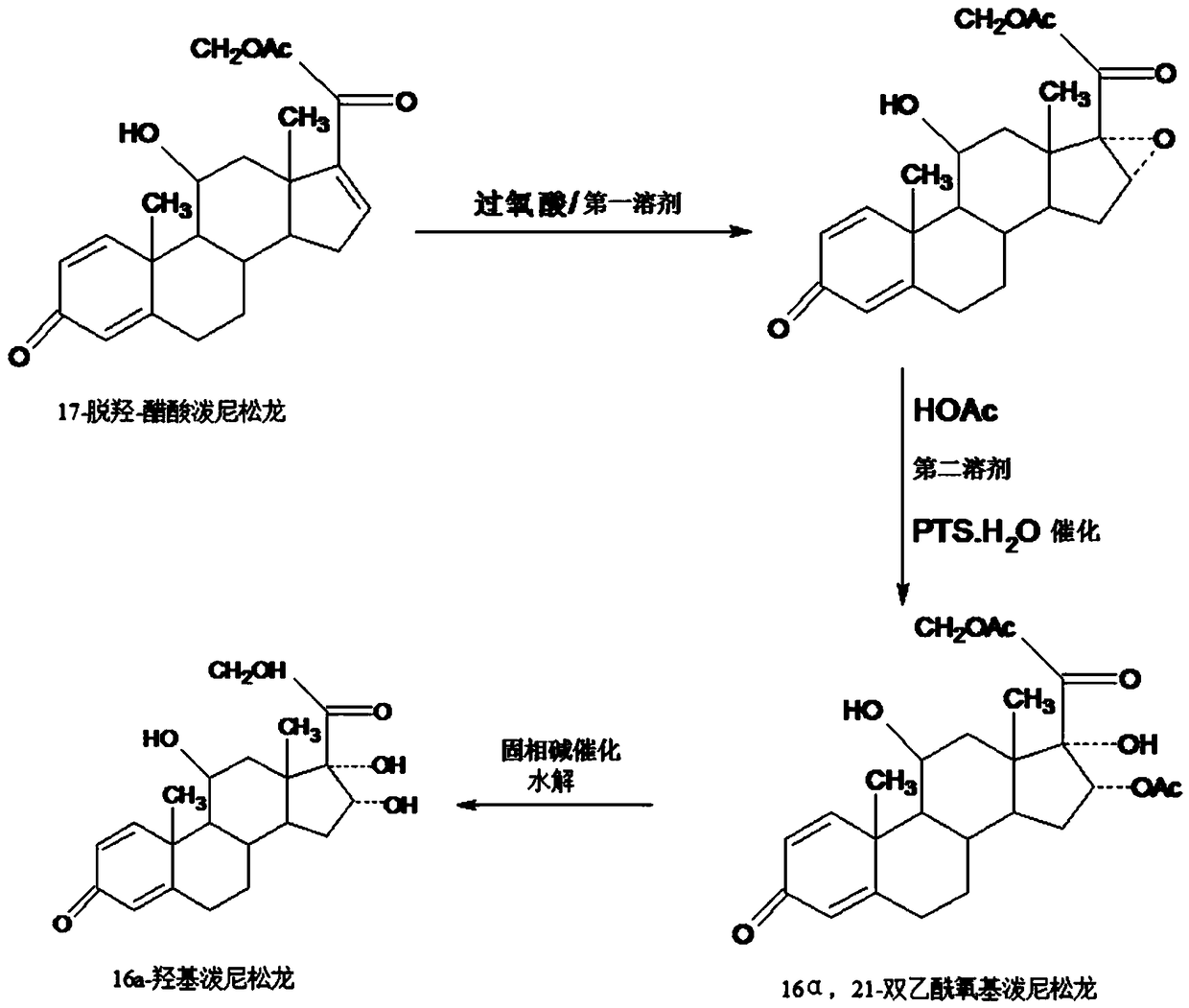
[0043] Step A---preparation of epoxy:
[0044] In a 3000ml three-necked flask, add 100g of starting materials 17a-prednisolone dehydrogenacetate, 1000ml of ethyl acetate, stir at room temperature to completely dissolve the solid, and add dropwise 40g of ophthalmic acid at 25-30°C The peracid solution made of oxybenzoic acid and 500ml ethyl acetate is dropped in about 2-2.5 hours, and then kept at 25-30°C for 6-12 hours, TLC confirms the end point of the reaction, and the reaction is complete Finally, add a mixed solution of 400ml 5% sodium hydroxide and 100ml 10% sodium sulfite to neutralize and destroy the acid produced by the reaction and excess peroxyacid, then concentrate under reduced pressure below 50°C to recover 90-95% ethyl acetate ester, then lowered to room temperature, added 500ml of tap water, stirred and analyzed for 3-4 hours, filtered, the filtrate was discharged into the wastewater treatment tank, the filter cake was washed with water, and dried below 60°C to ...
Embodiment 2
[0053] Step A: Preparation of epoxy:
[0054] In a 3000ml three-necked flask, add 100g of starting material 17a-prednisolone dehydroxyacetate, 1000ml of chloroform, stir at room temperature to completely dissolve the solid, and add 40g of m-chloroperoxy The peracid solution made of benzoic acid and 500ml ethyl acetate was dropped in about 2-2.5 hours, and then kept at 25-30°C for 6-12 hours, and the end point of the reaction was confirmed by TLC. After the reaction, add Mixed solution of 400ml 5% sodium hydroxide and 100ml 10% sodium sulfite to neutralize and destroy the acid and excess peroxyacid, then concentrate under reduced pressure below 50°C to recover 90-95% chloroform and ethyl acetate The mixed solvent was lowered to room temperature, added 500ml of tap water, stirred and analyzed for 3-4 hours, filtered, the filtrate was discharged into the waste water treatment tank, the filter cake was washed with water, and dried below 60°C to obtain 16,17a-epoxyprednilacetate S...
Embodiment 3
[0063] Step A---preparation of epoxy:
[0064] In a 3000ml three-necked flask, add 100g of starting materials 17a-prednisolone dehydrogenacetate, 1000ml of DME, stir at room temperature to completely dissolve the solid, and add dropwise a solution containing 40g of peracetic acid and 500ml of DME at 25-30°C. The prepared peracid solution was dripped in about 2-2.5 hours, and then continued to keep warm at 25-30°C for 6-12 hours after the dripping, and TLC confirmed the reaction end point. After the reaction, add 400ml of 5% sodium hydroxide and 100ml of a mixed solution made of 10% sodium sulfite to neutralize and destroy the acid and excess peroxyacid produced by the reaction, then concentrate under reduced pressure below 50°C to recover 90-95% DME solvent, then lower it to room temperature, add 500ml of tap water, Stir and analyze in water for 3-4 hours, filter, discharge the filtrate into the waste water treatment tank, wash the filter cake with water, and dry below 60°C to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com