A kind of preparation 16a, the method for 21-diacetyloxy prednisolone product
A technology of diacetoxyprednisolone and deshydroxyprednisolone, which is applied in the field of preparation of 16a, and achieves the effects of economical and environmentally friendly production process, reduced preparation cost, and avoiding many side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
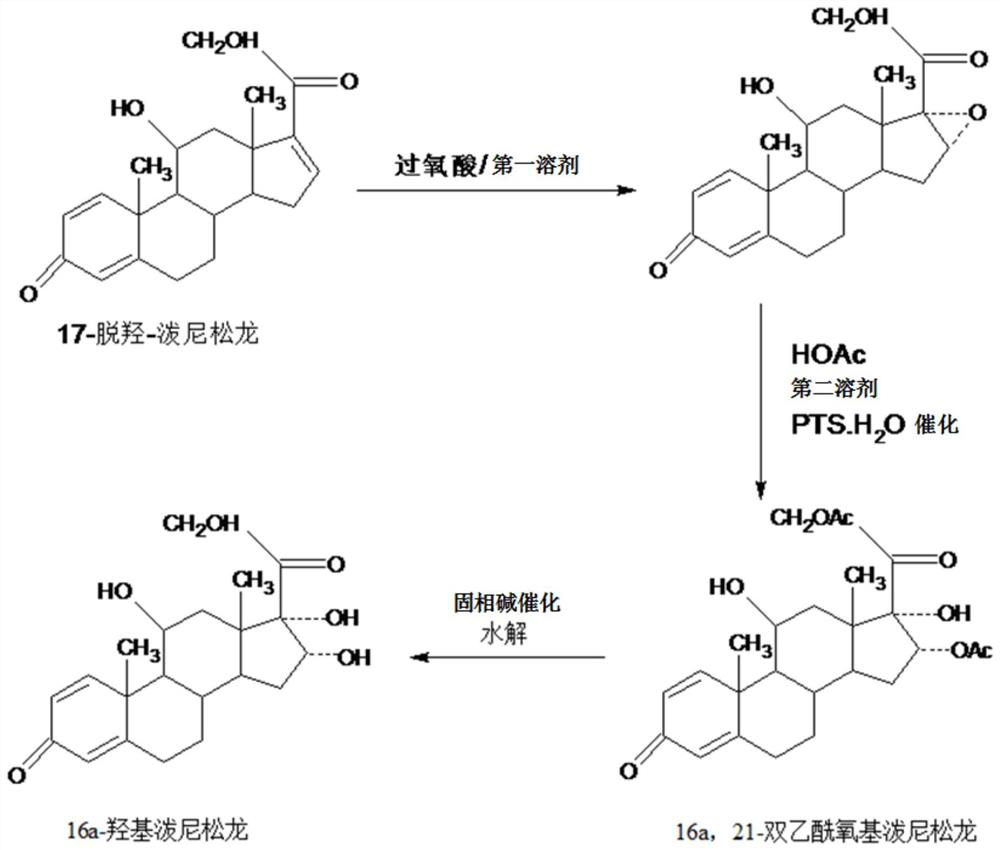
[0037] Step A---preparation of epoxy:
[0038] In a 3000ml three-neck flask, add 100g of starting material 17a-dehydroxyprednisolone, 1000ml of ethyl acetate, stir at room temperature to completely dissolve the solid, and add 40g of o-peroxy The peracid solution made of phthalic acid and 500ml ethyl acetate should be dripped in about 2-2.5 hours, and then keep warm at 25-30°C for 6-12 hours. TLC confirms the end point of the reaction. , add a mixed solution of 400ml 5% sodium hydroxide and 100ml 10% sodium sulfite to neutralize and destroy the acid produced by the reaction and excess peroxyacid, then concentrate under reduced pressure below 50°C to recover 90-95% ethyl acetate , then lowered to room temperature, added 500ml of tap water, stirred and analyzed for 3-4 hours, filtered, the filtrate was discharged into the wastewater treatment tank, the filter cake was washed with water, and dried below 60°C to obtain 99.3g of 16,17a-epoxyprednisolone , HPLC content 97.8%, weight...
Embodiment 2
[0047] Step A: Preparation of epoxy:
[0048] In a 3000ml three-necked flask, add 100g of starting material 17a-dehydroxyprednisolone, 1000ml of chloroform, stir at room temperature to completely dissolve the solid, and add 40g of m-chloroperoxybenzene dropwise under temperature control at 25-30°C For the peracid solution made of formic acid and 500ml ethyl acetate, drop it in about 2-2.5 hours, then continue to keep warm at 25-30°C for 6-12 hours, and confirm the end point of the reaction by TLC. After the reaction, add 400ml A mixed solution of 5% sodium hydroxide and 100ml 10% sodium sulfite to neutralize and destroy the acid and excess peroxyacid produced by the reaction, then concentrate under reduced pressure below 50°C to recover 90-95% of chloroform and ethyl acetate Mix the solvent, then lower it to room temperature, add 500ml of tap water, stir and analyze for 3-4 hours, filter, discharge the filtrate into the wastewater treatment tank, wash the filter cake with wate...
Embodiment 3
[0057] Step A---preparation of epoxy:
[0058] In a 3000ml three-necked flask, add 100g of starting material 17a-dehydroxyprednisolone and 1000ml of DME, stir at room temperature to completely dissolve the solid, and add dropwise a mixture of 40g of peracetic acid and 500ml of DME at 25-30°C under temperature control. The resulting peracid solution was dropped in about 2-2.5 hours, and then continued to insulate and react at 25-30°C for 6-12 hours. TLC confirmed the reaction end point. After the reaction, add 400ml 5% sodium hydroxide and 100ml A mixed solution made of 10% sodium sulfite is used to neutralize and destroy the acid and excess peroxyacid produced by the reaction, then concentrate under reduced pressure below 50°C to recover 90-95% DME solvent, then lower it to room temperature, add 500ml of tap water, and stir Water analysis for 3-4 hours, filtration, the filtrate was discharged into the waste water treatment tank, the filter cake was washed with water, and dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com