A quality detection method for concentrated Huoxin pills
A quality inspection method and technology for Huoxin Pills, which can be applied to measurement devices, instruments, scientific instruments, etc., and can solve the problems of insufficient comprehensiveness and one-sidedness of concentrated Huoxin Pills.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] The quality detection method of described concentrated heart pill comprises following identification method and assay method:
[0045] (1) Identification method
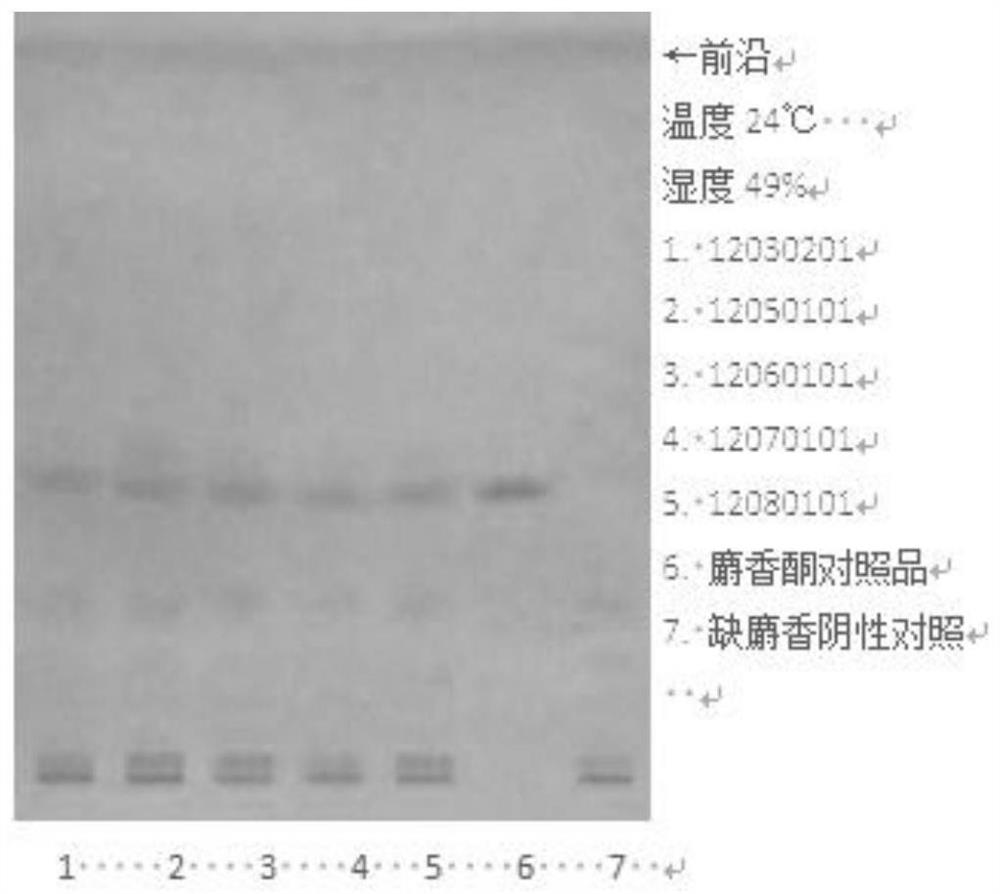
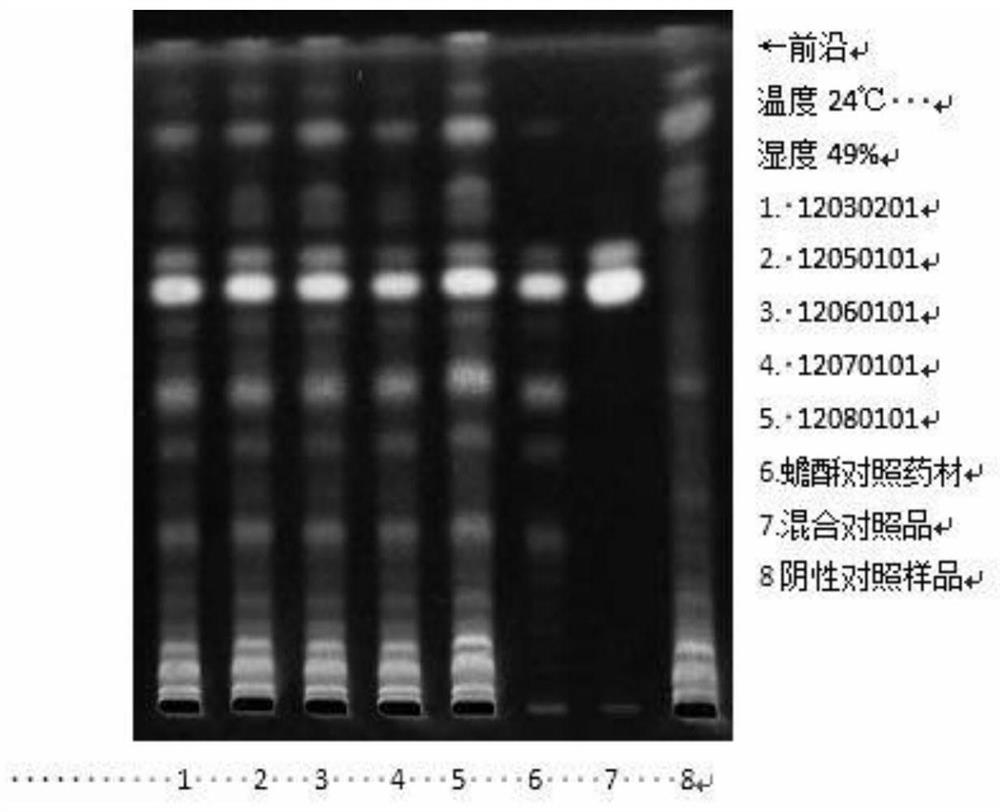
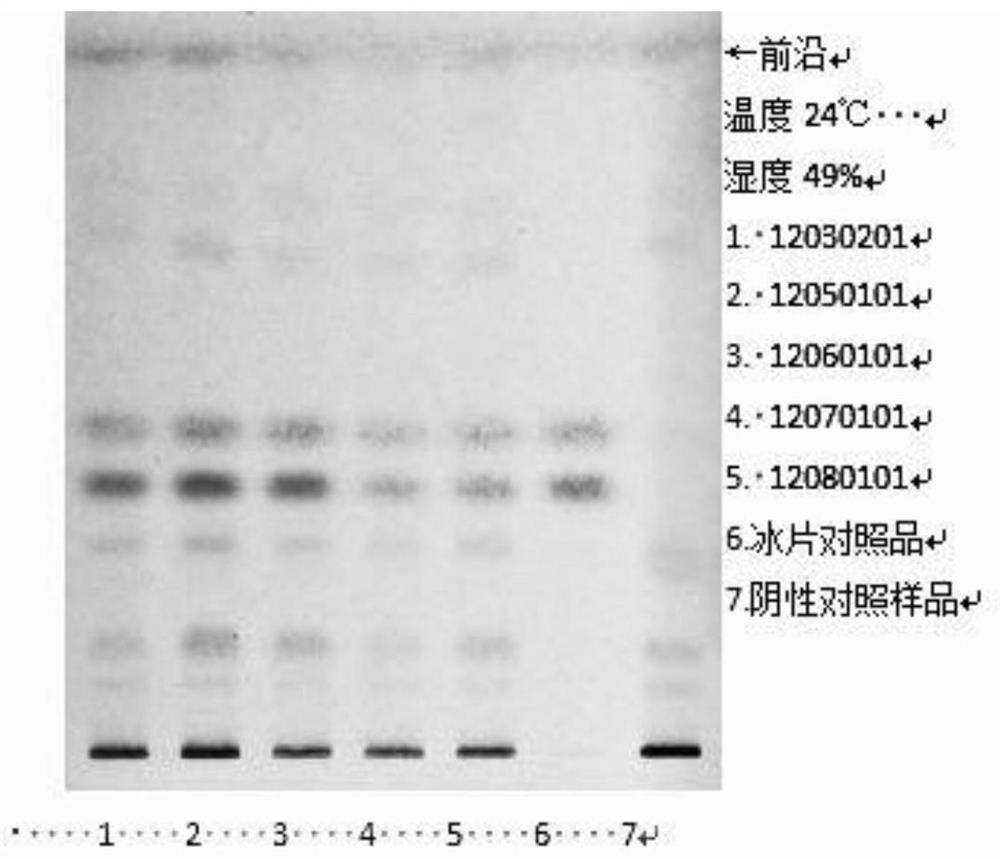
[0046] 1. TLC identification of muscone
[0047] ①Materials and reagents: Merck silica gel G thin-layer plates. All other reagents were analytically pure.
[0048] ②Reference substance: Muscone reference substance, batch number 110719-201014, purchased from China Inspection Institute.
[0049] ③ Test product: Concentrated Huoxin Pills with batch numbers 12030201, 12050101, 12060101, 12070101, 12080101, provided by Guangzhou Yuekang Biopharmaceutical Co., Ltd.
[0050] Take 0.8g each of 5 batches of the test sample, grind it finely, add 15ml of ether, ultrasonic treatment for 15 minutes, filter, filter the residue for later use, evaporate the filtrate, add 1ml of ethanol to the residue to dissolve, and use it as the test solution; For the reference substance, add ethanol to make a solution containing 1mg pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com