Application of oligomannuronic acid in inhibition of total tau expression, phosphorylation and aggregation
A mannuronic acid and phosphorylation technology, applied in the field of biomedicine, can solve problems such as unsatisfactory drug efficacy, achieve the effects of inhibiting hyperphosphorylation and abnormal aggregation, preventing/treating tauopathies, and having less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
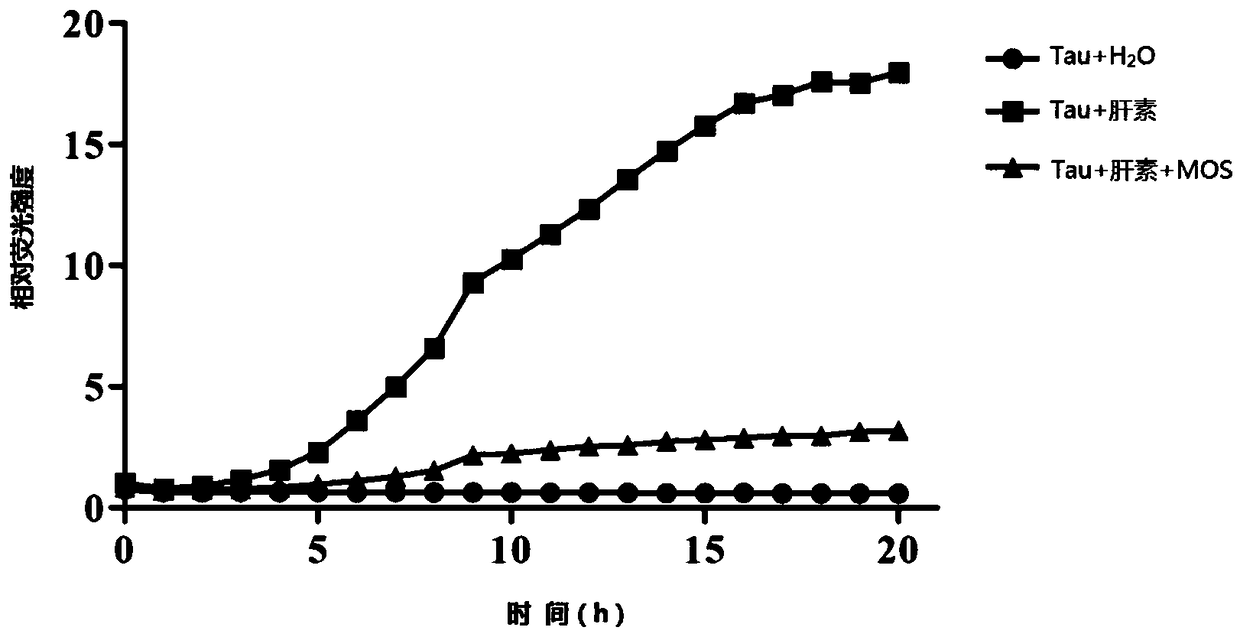
[0053] Example 1. The experiment of MOS inhibiting the aggregation of Tau protein induced by heparin in vitro:
[0054] Method: The oligomeric Tau protein was donated by Mr. Xiao Shifeng, School of Life and Marine Science, Shenzhen University. Tau protein + water, Tau protein + heparin were used as two groups of control groups, and the experimental group was: 200 μM oligomeric Tau protein was added to the black microplate, and then Thioflavin T, Tris-HCl buffer, heparin and 1mg / ml MOS. Using a full-wavelength fluorescent microplate reader, under the emission light of 440nm and the excitation light of 485nm, continuous detection was carried out for 20h, and the fluorescence values of each group were obtained, and the fluorescence values were used for drawing.
[0055] Results: Through this test, we know that in vitro, using heparin to induce the aggregation of Tau protein, adding MOS to the system can effectively inhibit the occurrence of Tau protein aggregation. The resu...
Embodiment 2
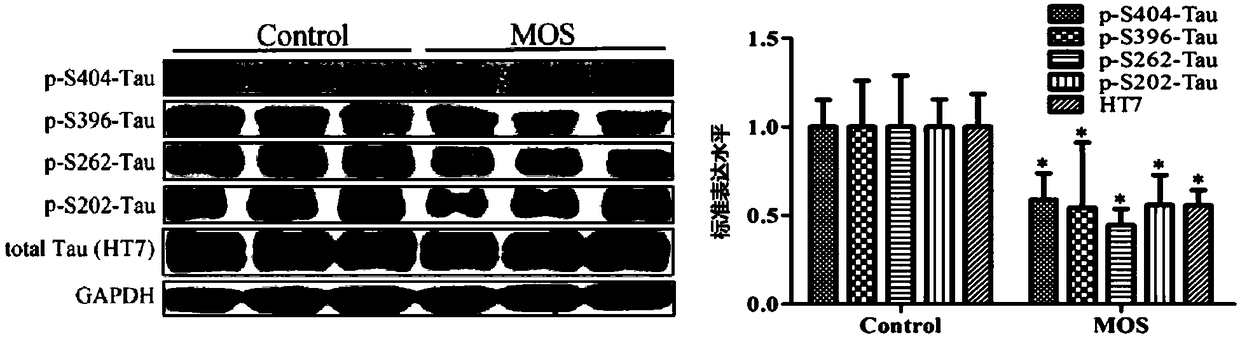
[0056] Example 2. MOS reduces the phosphorylation of Tau protein in primary neurons of HEK293 / Tau cells and TauP301L mice:
[0057] method:
[0058] a. MOS reduces the phosphorylation of Tau protein in HEK293 / Tau cells Method: Experimental group: HEK293 / Tau cells (2×10 6cells / well) attached to the wall in a 6-well plate, after 4-6 hours, discard the supernatant, add 1mg / ml MOS to treat for 24 hours, use cell lysate to extract total protein; control group: refer to the method of the experimental group, but do not add 1mg / ml MOS; the expression levels of total Tau and phosphorylated Tau protein detected by WesternBlot after completion.
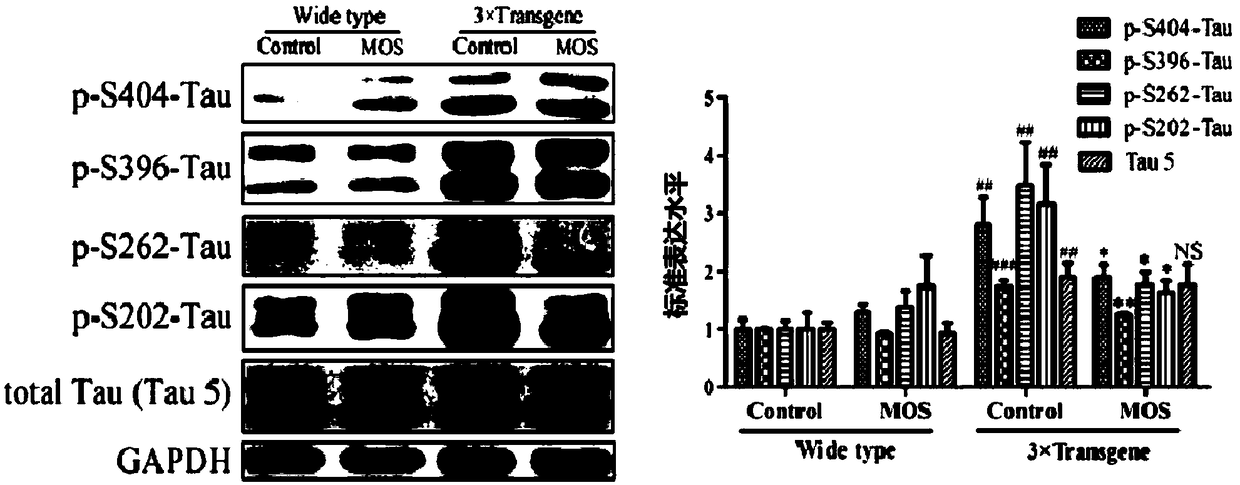
[0059] b. MOS reduces the phosphorylation of Tau protein in the primary neurons of TauP301L mice. Method: Experimental group: Inoculate the primary neurons of TauP301L mice and wild-type mice on a 6-well plate (7×10 5 per hole), add 1mg / ml MOS to treat for 24h, use cell lysate to extract total protein; control group: refer to the method of th...
Embodiment 3
[0063] Example 3. Experiment of MOS inhibiting GSK-3β activity in primary neurons of TauP301L mice:
[0064] Method: Experimental group: the primary neurons of TauP301L mice and wild-type mice were inoculated on 6-well plates (7×10 5 per hole), after adding 1mg / ml MOS to treat for 24h, the effect of MOS on the phosphorylation levels of GSK-3β and Akt in the primary neurons of TauP301L mice was detected by Western Blot method; the control group: refer to the method of the experimental group, However, 1 mg / ml MOS was not added.
[0065] result:
[0066] Western Blot was used to detect the effect of MOS on the phosphorylation levels of GSK-3β and Akt in the primary neurons of TauP301L mice. Figure 4 shown. The experimental results showed that compared with the primary neurons of wild-type mice, the phosphorylation level of Akt in primary neurons of TauP301L mice was decreased, and the phosphorylation level of total GSK-3β and its tyrosine 216 site was increased. High, the ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com