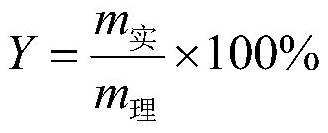The preparation method of 3,5-dichloro-2-pentanone
A technology of pentanone and sulfonyl chloride, which is applied in the field of preparation of 3,5-dichloro-2-pentanone, can solve the problems of complex process and non-environmental protection, and achieve the effects of simple process, avoiding three wastes, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0008] The invention provides a preparation method of 3,5-dichloro-2-pentanone, wherein the preparation method comprises: contacting α-acetyl-γ-butyrolactone and sulfonyl chloride in a system without solvent A chlorination reaction is carried out, and then the material obtained by the chlorination reaction is mixed with water, and hydrochloric acid is added dropwise to the obtained mixture to carry out a ring-opening reaction.
[0009] According to the present invention, in order to improve the yield of 3,5-dichloro-2-pentanone and reduce production cost, preferably, in the chlorination reaction, the consumption of α-acetyl-γ-butyrolactone is the same as The molar ratio of the amount of sulfonyl chloride is 1:1-1.5, preferably 1:1-1.05.
[0010] According to the present invention, the conditions of the chlorination reaction preferably include: the reaction temperature is 0-60°C, preferably 0-20°C; the reaction time is 0.4-4h, preferably 0.5-2h. Under the preferred chlorinatio...
Embodiment 1
[0028] Add 261.5g (2mol) of α-acetyl-γ-butyrolactone to a 1000ml four-necked bottle, control the temperature at 15°C, add 281g (2.04mol) of sulfonyl chloride dropwise, the dropping rate is 4.08mol / h, continue to Stir under temperature control for 1 h until the conversion of raw materials is complete, then add 100 g of water. Then, the temperature was raised to 100° C., and 344.7 g of 36% by weight hydrochloric acid (3.4 mol of HCl) was added dropwise at a rate of 3.4 mol / h in terms of HCl. After the drop was completed, the temperature-controlled stirring was continued for 3 h. After the reaction was completed, it was lowered to room temperature, and then 500 g of 1,2-dichloroethane was added to the system for extraction and separation. The organic layer was distilled under reduced pressure to obtain 3,5-dichloro-2-pentanone with a purity of 98%. The yield is 90%.
Embodiment 2
[0030] Add 261.5g (2mol) of α-acetyl-γ-butyrolactone into a 1000ml four-necked bottle, control the temperature at 20°C, add 289g (2.1mol) of sulfonyl chloride dropwise, and the dropping rate is 2.1mol / h, continue to Stir under temperature control for 0.5 h until the conversion of raw materials is complete, then add 209 g of water. Then, the temperature was raised to 110° C., and 1217 g of 15% by weight hydrochloric acid (5 mol of HCl) was added dropwise at a rate of 2.5 mol / h in terms of HCl. After the drop was completed, the temperature-controlled stirring was continued for 6 h. After the reaction was completed, it was lowered to room temperature, and then 500 g of 1,2-dichloroethane was added to the system for extraction and separation. The organic layer was distilled under reduced pressure to obtain 3,5-dichloro-2-pentanone with a purity of 98%. The yield was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com