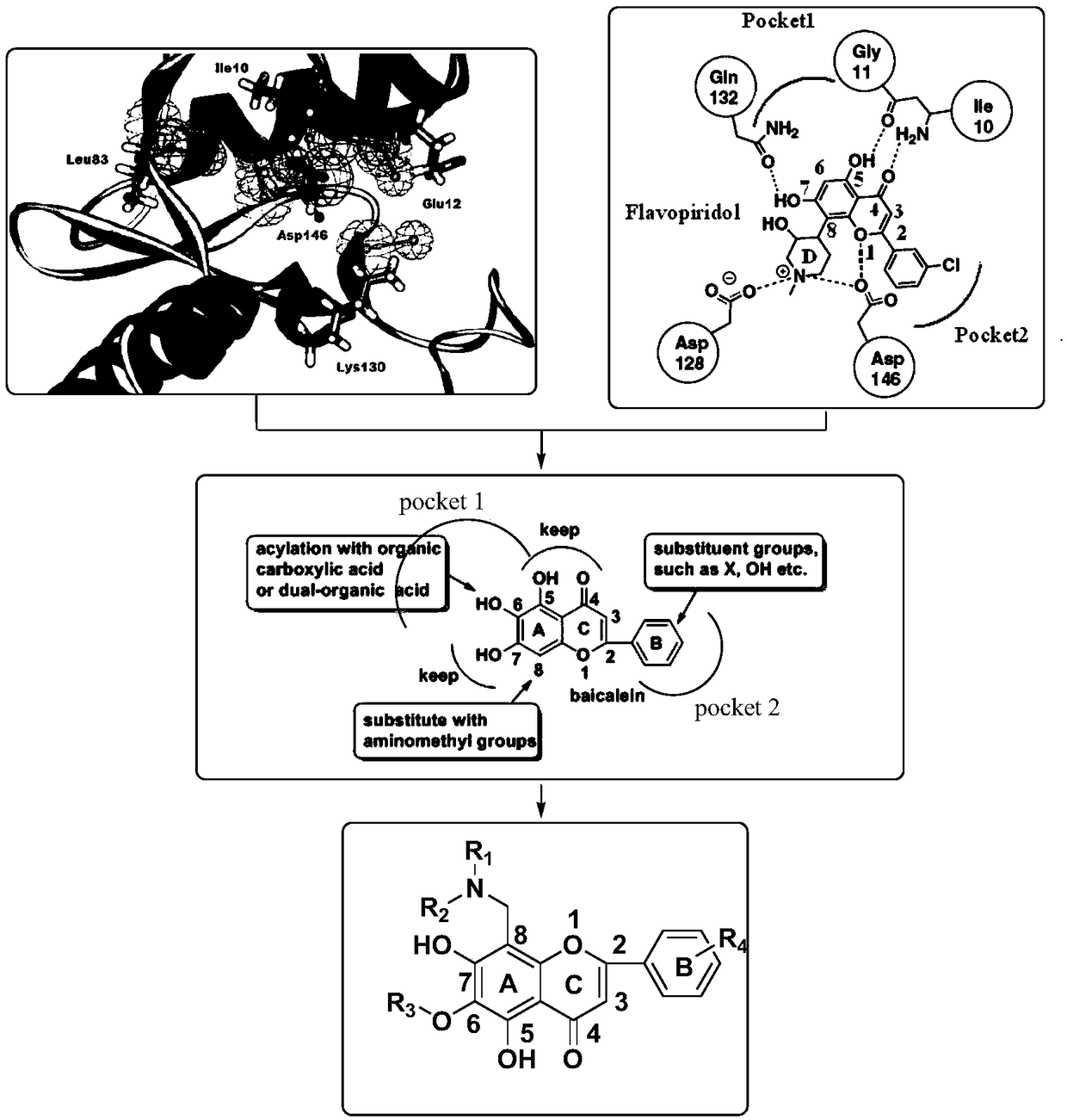
Flavonoid derivative as well as preparation method and identification method thereof
An identification method and a derivative technology, which are applied in the field of flavonoid derivatives and their preparation, can solve the problem that the crystal structure of CDK1 has not been reported, and achieve the effects of improving selective inhibition and clear target.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
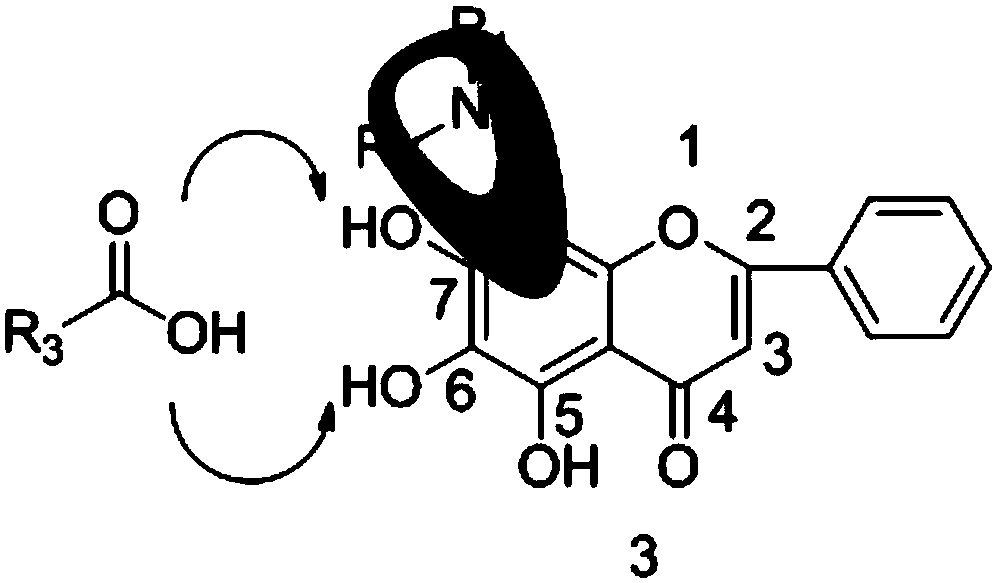
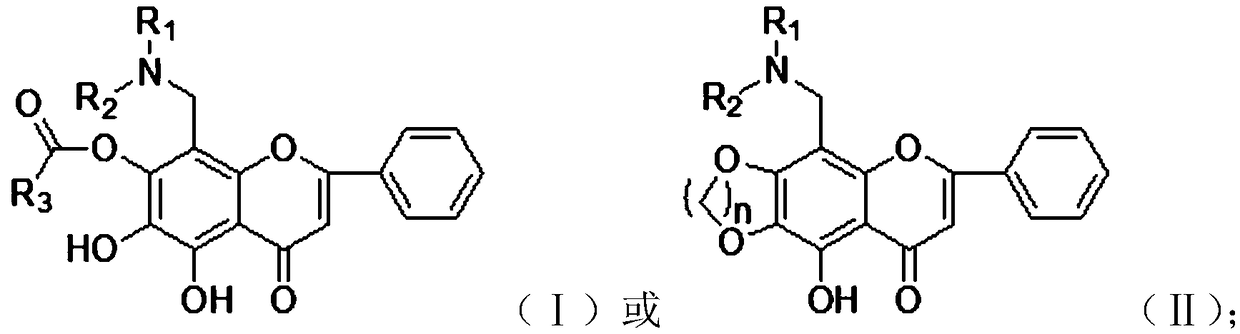
[0058] The present invention also provides a preparation method of flavonoid derivatives, using a compound of general formula (III) to obtain a compound of general formula (IV) after deglucuronidation, and then substituting an aminomethyl group to obtain a compound of general formula ( V) compounds;
[0059] Carry out esterification reaction to the compound with general formula (V) to obtain the compound with general formula (I);
[0060] Carry out etherification reaction to the compound with general formula (V) to obtain the compound with general formula (II);
[0061] Wherein, the compound with general formula (III), general formula (IV) and general formula (V) is:
[0062]
[0063] Among them, R 1 is alkyl or cycloalkyl, R 2 is alkyl or cycloalkyl, R 1 , R 2 and a nitrogen atom to form an alicyclic or heterocyclic ring.
[0064] In another embodiment, R 1 , R 2 and nitrogen atom to form a tetrahydropyrrole ring.
[0065] In another embodiment, R 1 , R 2 and ni...
Embodiment 1
[0079]
[0080] Weigh 4 g of baicalin (compound represented by formula (Ⅲ)) and dissolve it in 20 mL of water to form a paste, slowly add 100 mL of 50% sulfuric acid, mechanically stir in a water bath at 90°C until clear, continue to react for 5 minutes, and pour it into the preparation Prepare 500mL of ice water, then carry out suction filtration under reduced pressure, wash the filter cake with water until nearly neutral, discard the filtrate, dry the filter cake under vacuum for 24 hours, take appropriate amount of ethyl acetate to disperse into a homogeneous suspension, and filter under reduced pressure , the filter cake was washed 3 times with ethyl acetate, and the filter cake was vacuum-dried for 24 hours to obtain 1.74 g of baicalein (compound represented by formula (IV)) with a yield of 71.9%.
[0081] Weigh 1.08g (0.004mol) of baicalein (the compound shown in formula (IV)) in a 150mL reaction flask, add 30mL of methanol as a solvent, and add 0.486mL (0.006mol) of 3...
Embodiment 2
[0089]
[0090] Weigh 4 g of baicalin (the compound represented by formula (III)) and dissolve it in 20 mL of water to form a paste, slowly add 100 mL of 50% sulfuric acid, stir mechanically in a water bath at 90°C until it becomes clear, continue to react for 5 minutes, and pour into the preparation while stirring. 500mL of fresh ice water, then subjected to vacuum filtration, the filter cake was washed with water until nearly neutral, the filtrate was discarded, and after the filter cake was vacuum-dried for 24 hours, an appropriate amount of ethyl acetate was dispersed into a homogeneous suspension, and vacuum filtered. , the filter cake was washed three times with ethyl acetate, and the filter cake was vacuum-dried for 24 h to obtain 1.74 g of baicalein (a compound represented by formula (IV)) with a yield of 71.9%.
[0091] Weigh 1.08 g (0.004 mol) of baicalein (a compound represented by formula (IV)) in a 150 mL reaction flask, add 30 mL of methanol as a solvent, and u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com