Prolyl hydroxylase small molecular fluorescent probes and preparation method thereof
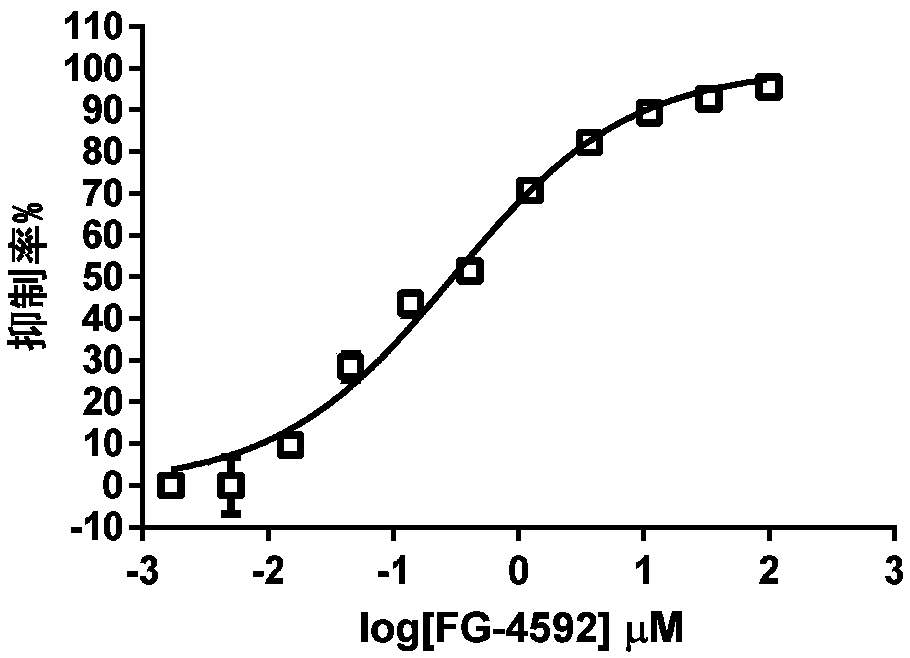
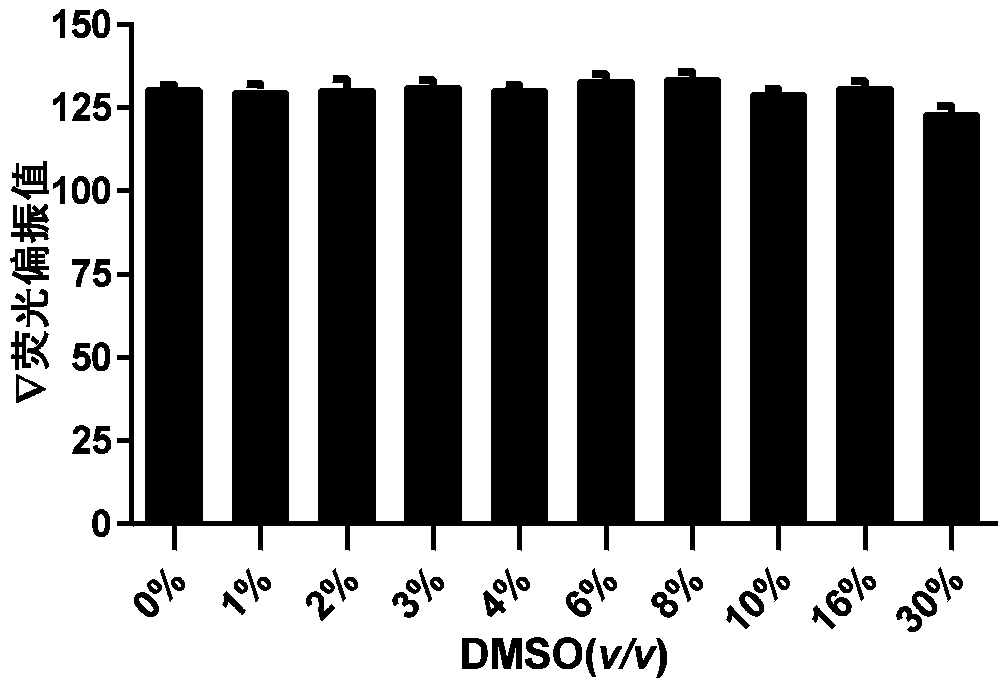
A technology of prolyl hydroxylase and fluorescent probe, which is applied in chemical instruments and methods, fluorescence/phosphorescence, analytical materials, etc., can solve the problems of complex test system, loss of binding ability of PHD enzyme, and decrease of binding ability of HIF fluorescent peptide and other problems, to achieve the effect of simple test system and optimized structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of fluorescent probe Ⅰ-1
[0065] (3-((2-(2-azidoethoxy)-5-fluorophenyl)amino)propyl)carbamate tert-butyl ester (0.706g, 2.0mmol), (5-ethynyl-3- Hydroxypicolilyl) glycine (0.440g, 2.0mmol), tetrabutylammonium fluoride (1.0mol / L solution in THF, 1.56g, 6.0mmol), cuprous iodide (0.023g, 0.12mmol) and N, N-Diisopropylethylamine (1.0mL) was dissolved in MeOH (8mL) under N 2 The mixture was stirred at 80° C. for 5.5 hours under protection, and the reaction mixture was filtered and concentrated. The product was purified by column chromatography on silica gel (eluent: 30-50% EtOAc in petroleum ether) to afford (5-(1-(2-(2-((3-((tert-butoxycarbonyl)amino) ) Propyl) amino)-4-fluorophenoxy) ethyl)-1H-1,2,3-triazol-4-yl)-3-hydroxypyridinecarbonyl)glycine 0.620g; Soluble in anhydrous CH 2 Cl 2 (5 mL), TFA (1 mL) was added dropwise to the reaction solution, and after stirring at room temperature for 3 hours, the reaction mixture was concentrated in vacuo. Dissolve ...
Embodiment 2
[0067] Preparation of fluorescent probe Ⅰ-2
[0068] The preparation method is the same as in Example 1, with (7-((2-(3-azidopropoxy)-5-chlorophenyl)amino)heptyl)carbamate tert-butyl ester (0.878g, 2.0mmol) replacing (3-((2-(2-azidoethoxy)-5-fluorophenyl)amino)propyl)carbamate tert-butyl ester, 0.524g of yellow solid was obtained, the three-step yield was 27.6%, R f : 0.11 (methanol:ethyl acetate=2:5), m.p.159.7-160.1°C, the compound 1 H NMR (500MHz, DMSO-d 6 )δ9.10(s,1H),8.87(d,J=1.3Hz,1H),8.34(s,1H),8.20(s,1H),7.75–7.69(m,2H),7.65(d,J =2.0Hz,1H),7.27–7.19(m,2H),7.14(dd,J=10.2,7.4Hz,2H),7.01(dd,J=7.5,2.0Hz,1H),7.00(s,2H) ,6.78–6.70(m,2H),6.64–6.57(m,2H),6.50(d,J=2.1Hz,1H),6.34(dd,J=7.5,2.0Hz,1H),4.55(td,J =12.4,1.9Hz,1H),4.40(s,1H),4.23(d,J=12.3Hz,1H),4.14(d,J=12.4Hz,1H),3.91(ddt,J=20.2,12.2, 2.9Hz, 2H), 3.69–3.57(m, 1H), 3.40(dd, J=12.4, 10.9Hz, 1H), 3.28(td, J=12.0, 1.2Hz, 1H), 2.90(td, J=12.2 ,4.2Hz,1H),2.29–2.18(m,1H),2.04–1.54(m,6H),1.53–1.43(m,2H),1.44–1.25(m,2H)....
Embodiment 3
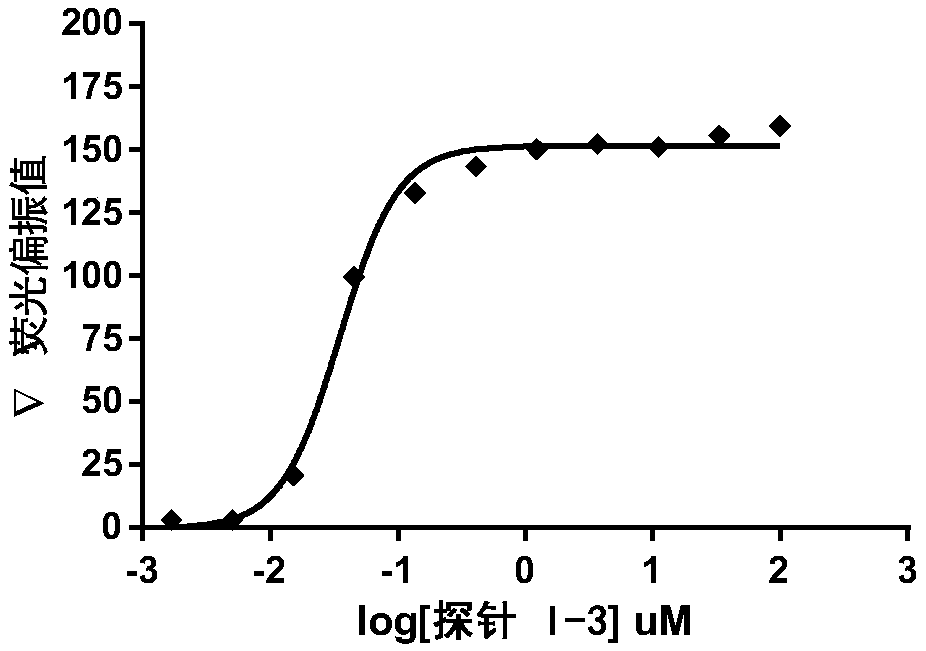
[0070] Preparation of fluorescent probe Ⅰ-3
[0071] The preparation method is the same as in Example 1, with (4-(2-(2-azidoethoxy)-5-iodophenoxy) butyl) tert-butyl carbamate (0.950g, 2.0mmol) replacing (3 -((2-(2-azidoethoxy)-5-fluorophenyl)amino)propyl)carbamate tert-butyl to give yellow solid 0.586g, three-step yield 29.8%, R f : 0.19 (methanol:ethyl acetate=1:2), m.p.161.7-162.8°C, the compound 1 H NMR (500MHz, DMSO-d 6 )δ9.59(s,1H),8.99(d,J=1.3Hz,1H),8.57(s,1H),8.20(s,1H),7.72(dd,J=7.5,2.0Hz,1H), 7.68–7.61(m,3H),7.42–7.34(m,2H),7.30(s,1H),7.16–7.10(m,1H),7.00(s,2H),6.85(dd,J=19.8,7.5 Hz,2H),6.52(dd,J=7.4,1.9Hz,1H),6.38(s,1H),5.09–4.96(m,2H),4.79–4.65(m,2H),4.58(ddt,J= 12.5,4.8,1.4Hz,1H), 4.36(ddd,J=12.6,11.4,4.0Hz,1H),4.17–4.08(m,2H),4.01–3.89(m,2H),2.32(qt,J= 12.4,3.3Hz,1H),2.21–2.10(m,1H),2.06–1.95(m,1H),1.81(dd,J=12.2,8.6,Hz,1H)., 13 C NMR (125MHz, DMSO-d 6 )δ179.64,172.72,170.00,168.45,158.76,153.94,151.93,150.97,149.24,146.85,146.41,142.66,139.43,137.31,136.03...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com