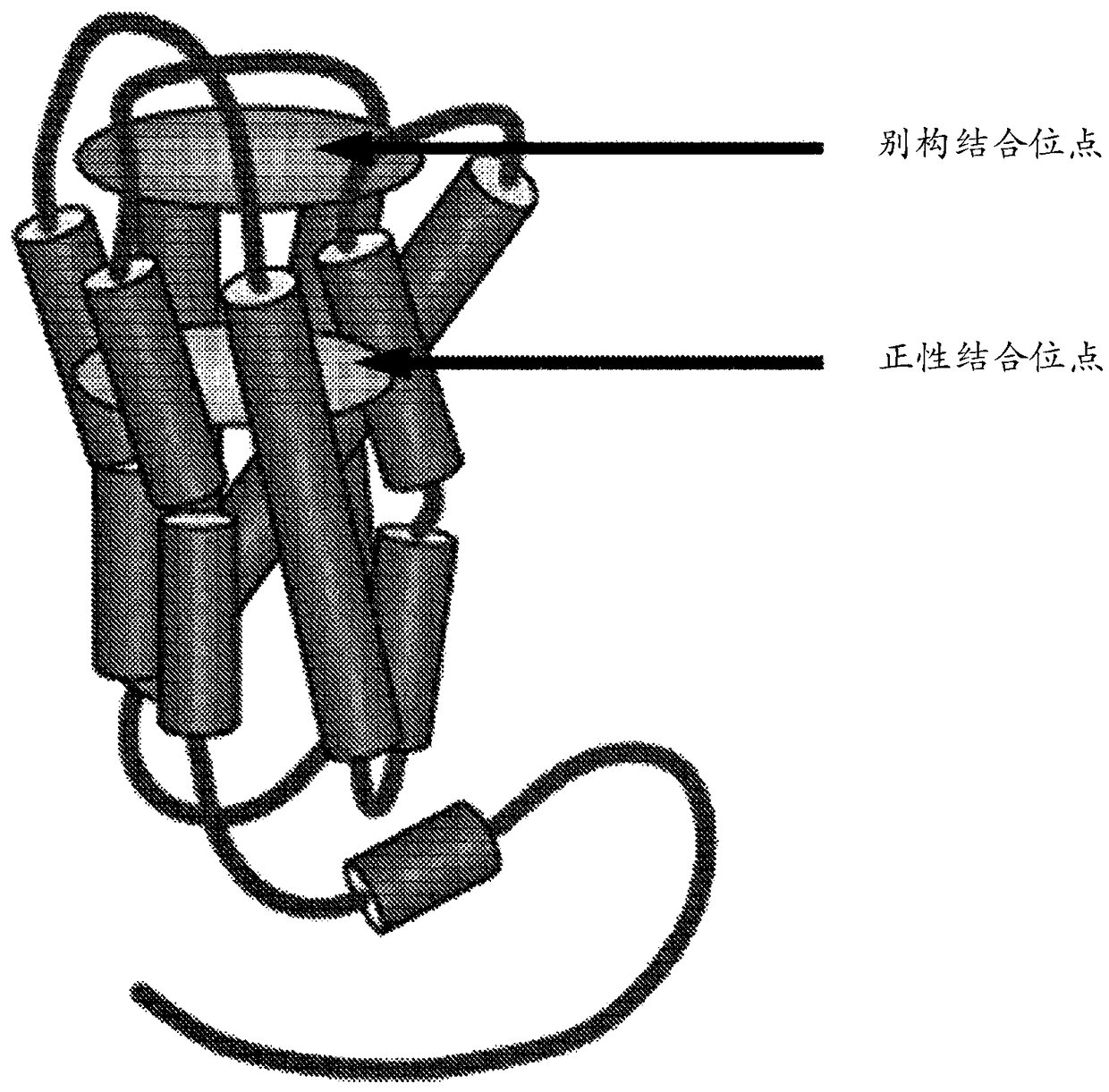
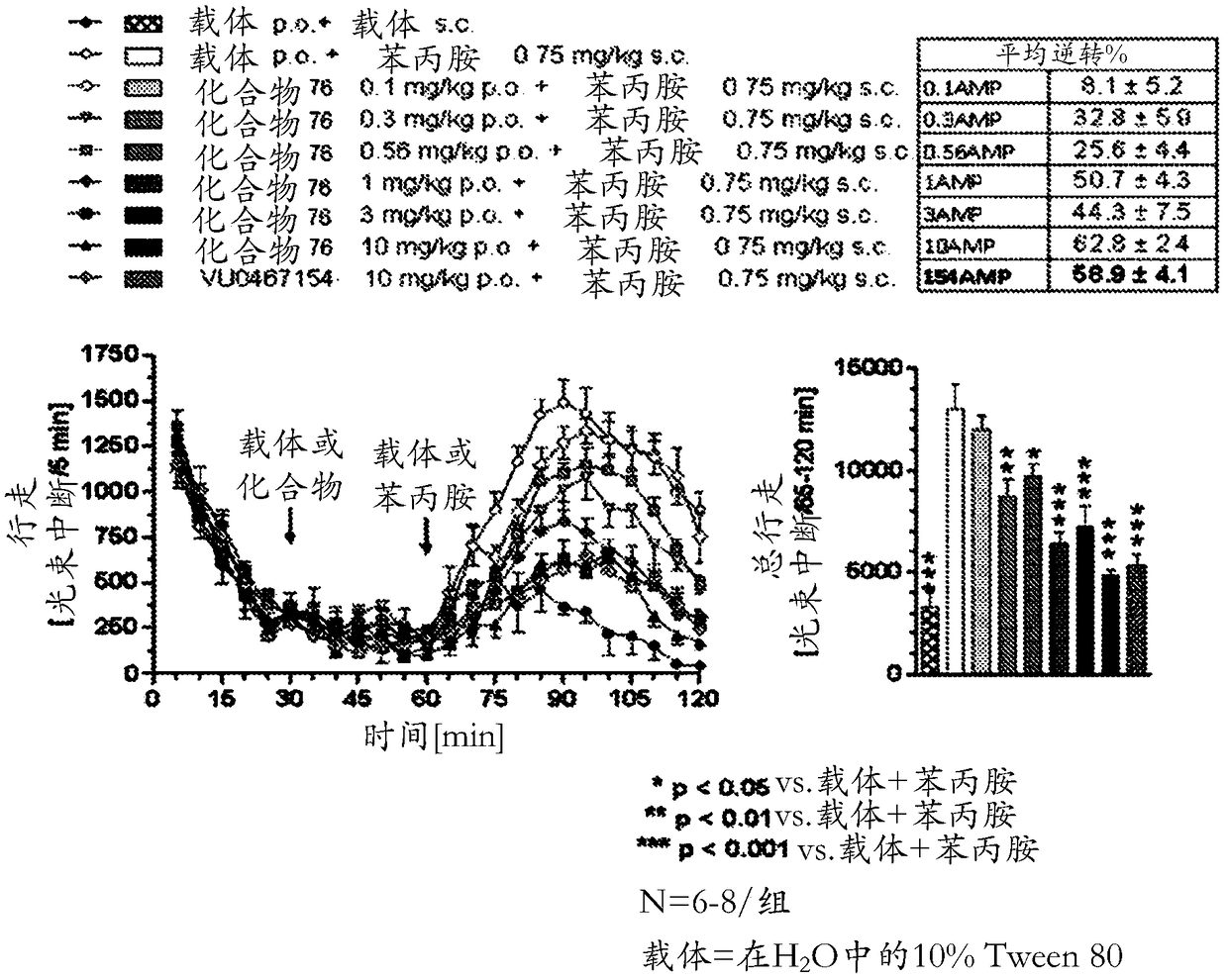
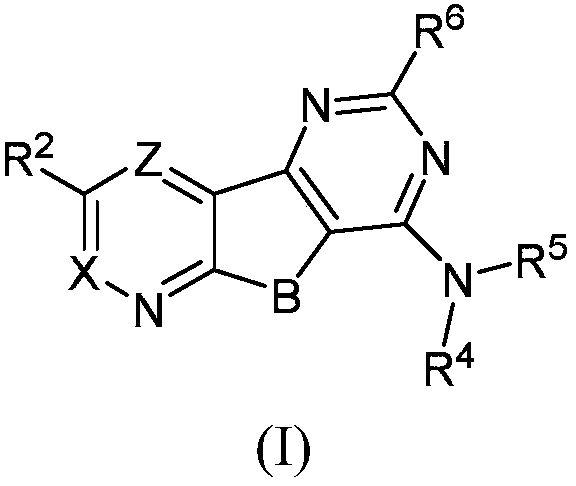
Positive allosteric modulators of the muscarinic acetylcholine receptor m4
A C1-C4, C1-C4- technology, applied in the field of positive allosteric modulators of muscarinic acetylcholine receptor M4, can solve problems such as limiting clinical utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0742] Example 1. General Amine Synthesis
[0743] The following are exemplary syntheses of certain amines useful in the preparation of the compounds disclosed herein.
[0744]
[0745] 4-(1-methoxy-1-methyl-ethyl)benzonitrile (X1). To a solution of 4.34 mmol, 1.0 equiv) in DMF (16.4 mL) was added sodium hydride (60% in mineral oil, 260 mg, 6.51 mmol, 1.5 equiv). After 1 h at 5 °C, iodomethane (0.30 mL, 4.78 mmol, 1.1 equiv) was added. The mixture was allowed to warm to room temperature. After 2 h, water (20 mL) was added followed by EtOAc (30 mL). The layers were separated. The aqueous layer was extracted with EtOAc (2 x 30 mL). The combined organic layers were washed with brine, dried (Na 2 SO 4 ), filtered and concentrated. The residue was purified by flash column chromatography on silica gel (0-50% EtOAc / hexanes) to afford the title compound (600 mg, 79% yield) as a viscous oil. ES-MS[M+1] + :176.6; 1 H NMR (400MHz, DMSO-d 6 ) δ 7.82 (ddd, J = 8.6, 1.8, 1.8H...
example 2
[0779] Example 2. N,N,3,4-Tetramethylpyrimido[4',5':4,5]thieno[2,3-c]pyridazin-8-amine (Compound 1)
[0780]
[0781] Ethyl 5-amino-3,4-dimethylthieno[2,3-c]pyridazine-6-carboxylate (A). To a 20 mL microwave vial was added 3-chloro-5,6-dimethyl- Pyridazine-4-carbonitrile (1.20 g, 7.16 mmol), potassium carbonate (1.98 g, 14.3 mmol), IPA (15 mL) and ethyl thioglycolate (0.87 mL, 7.88 mmol). After 20 min in the microwave reactor at 105 °C, the reaction was cooled to room temperature and added to water (150 mL). The solid was filtered and washed 3 times with water to give A (1.42 g, 79% yield) as a green powder. ES-MS[M+1] + :252.2; 1 H NMR (400MHz, CDCl 3 )δ 6.18 (s, 2H), 4.41 (q, J = 7Hz, 2H), 2.85 (s, 3H), 2.77 (s, 3H), 1.44 (t, J = 7.12Hz, 3H).
[0782] 3,4-Dimethylpyrimido[4',5':4,5]thieno[2,3-c]pyridazin-8-ol (B). Heat A in an open vessel at 150°C (1.41 g, 5.61 mmol) in formamide (10 mL, 252 mmol). Formamidine acetate (1.75 g, 16.8 mmol) was added to the reaction. ...
example 3
[0785] Example 3. 2-[4-[[(3,4-Dimethylpyrimido[4',5':4,5]thieno[2,3-c]pyridazin-8-yl)amino]methanol Base] phenyl] propan-2-ol (compound 76)
[0786]
[0787] 5-amino-3,4-dimethylthieno[2,3-c]pyridazine-6-carboxamide (A').To 5-amino-3,4-dimethyl-thieno[2, 3-c] Ethyl pyridazine-6-carboxylate (A, prepared as described in Example 2) (5.0 g, 19.9 mmol, 1.0 equiv) suspension in THF (33.0 mL) and MeOH (10.0 mL) Aqueous LiOH (1M, 99.5 mL, 5.0 equiv) was added to . After 16 hours at room temperature, LCMS confirmed loss of starting material. The reaction was cooled to 0 °C and the yellow solid was filtered off. The solid was dried in vacuo to afford lithium carboxylate (4.6 g) as a yellow powder. ES-MS[M+1] + :224.4; 1 H NMR (400MHz, DMSO-d 6 )δ6.37(s,2H),2.65(s,6). The filtrate was acidified with 4M HCl in dioxane to give the corresponding carboxylic acid (950 mg). ES-MS[M+1] + :224.3; 1 H NMR (400MHz, DMSO-d 6 )δ7.01 (bs, 2H), 2.73 (s, 3H), 2.71 (s, 3H).
[0788] To a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com