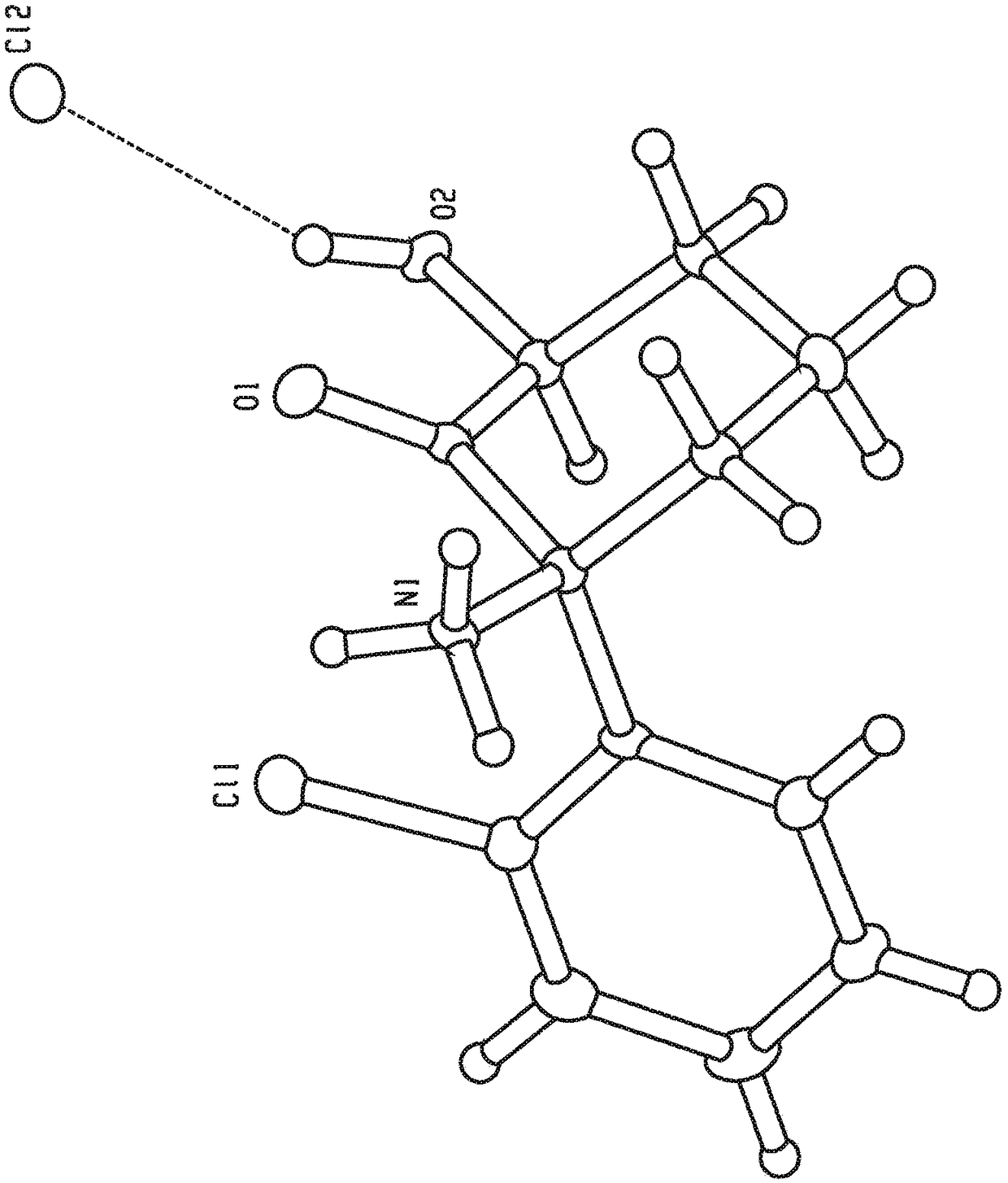
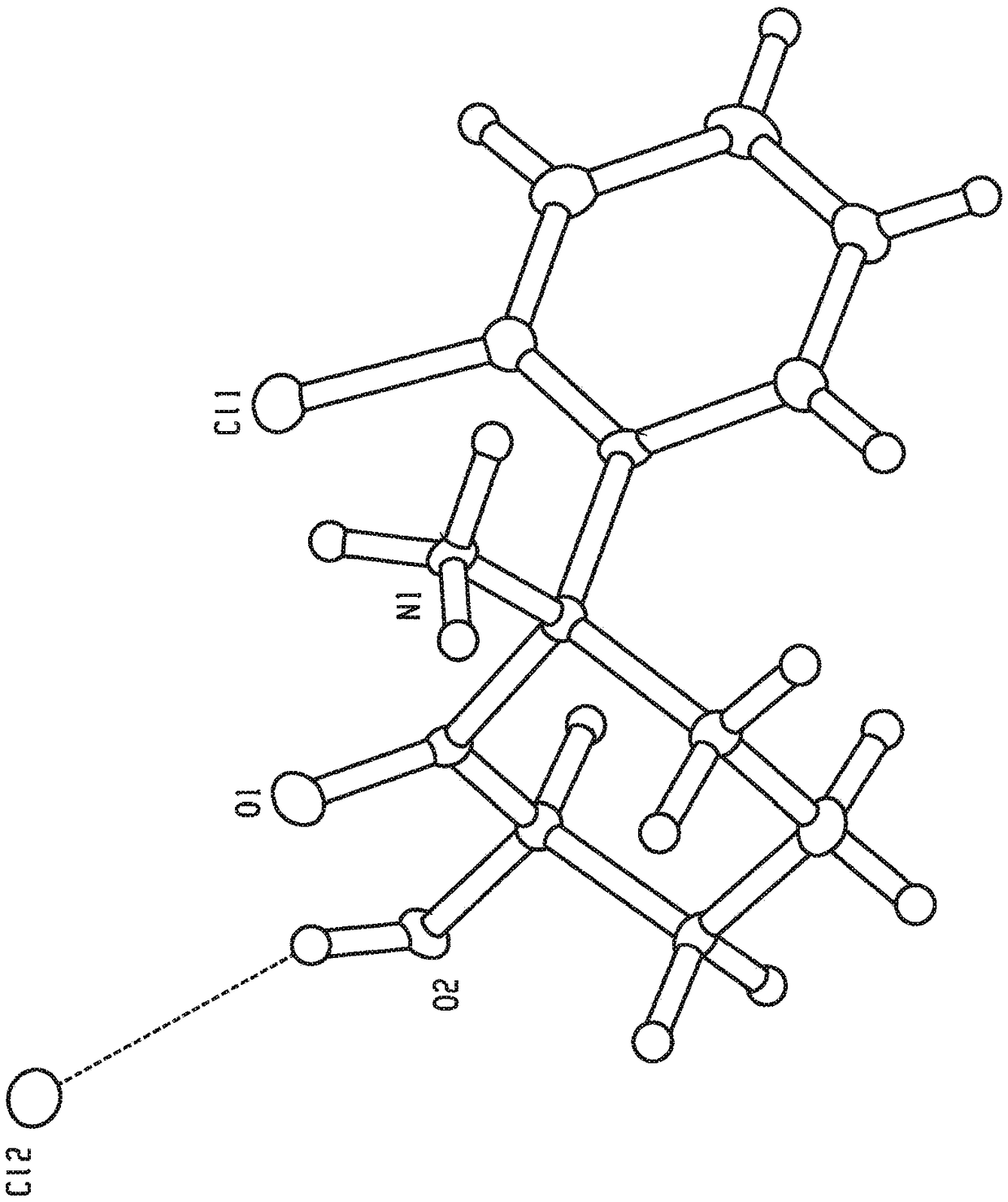
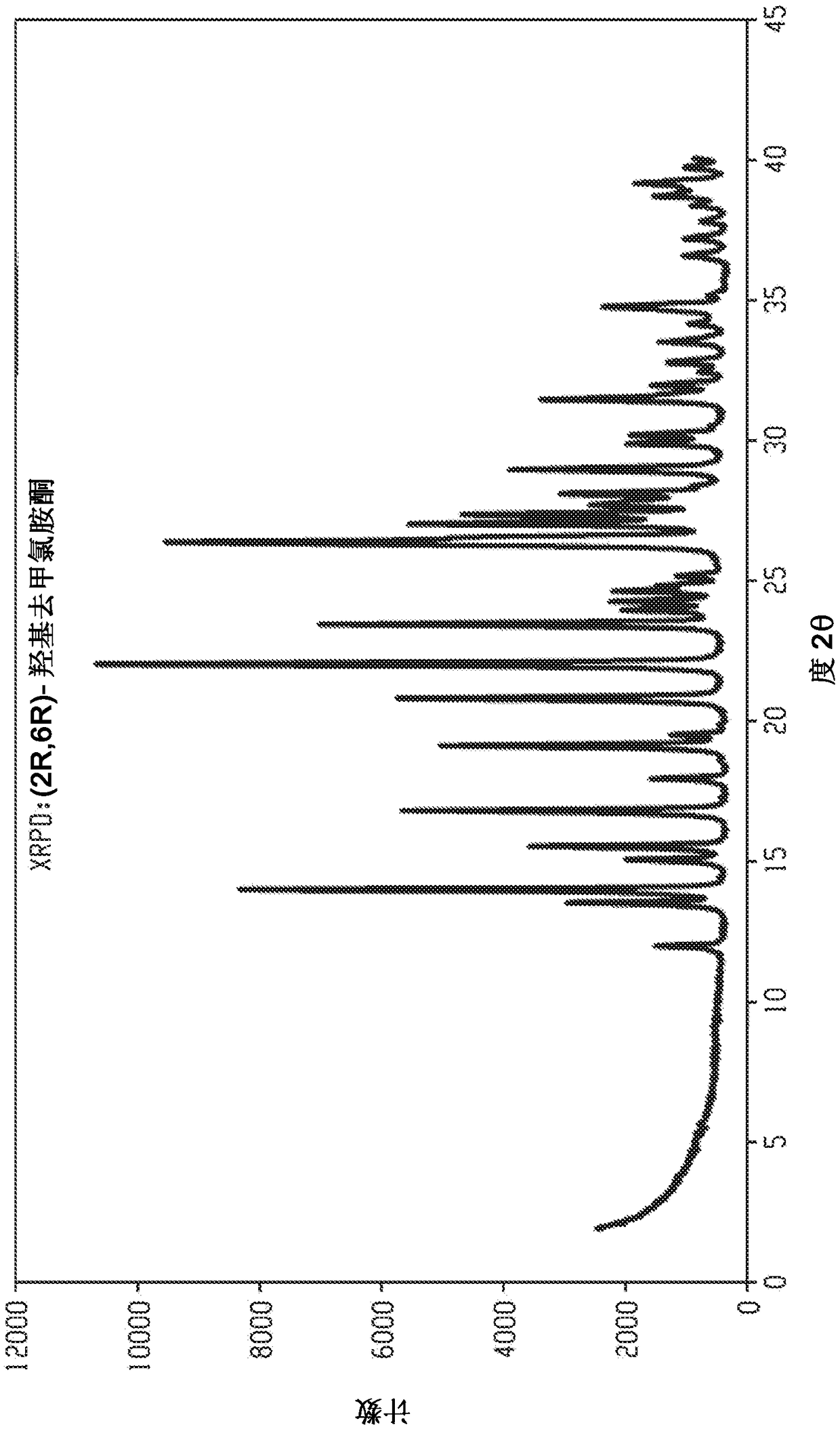
Crystal forms and methods of synthesis of (2r, 6r)-hydroxynorketamine and (2s, 6s)-hydroxynorketamine
A technology of methylketamine, methylketamine hydrochloride, applied in the crystal form of hydrochloride, recrystallizing 2R,6R-HNK hydrochloride, major depression, standard treatment selective 5-hydroxy, sexual biphasic Depression, as well as other areas of depression and focus, can address issues such as unresponsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1. Chiral resolution of (S)-(+)-norketamine (14)
[0158]
[0159] Racemic norketamine (22.7 g, 101 mmol) (Cayman Chemicals, Ann Arbor, MI, USA, prepared as described by Hong, S.C. & Davisson, J.N., J.Pharm.Sci. (1982) 71:912-914) Dissolve in 1.1L ethanol. Then solid (D)-(R)-(+)-pyroglutamic acid (15.8 g, 0.5 equiv, 121 mmol) was added. The reaction was stirred and heated to reflux for 5 minutes. A white suspension formed while heating. Once the suspension reached reflux, it was allowed to cool to room temperature while stirring for 16 hours. The reaction was filtered and a white solid was collected. The resulting white solid was then resuspended in 0.9 L of ethanol, and the suspension was heated to reflux for 5 minutes. The suspension was allowed to cool to room temperature over 2 hours while stirring. The solid was collected by filtration, then suspended a third time in ethanol (0.8 L), heated to reflux for 5 minutes, then allowed to cool to room temp...
Embodiment 2
[0160] Example 2. Chiral resolution of (R)-(-)-norketamine (14A)
[0161]
[0162] (R)-(-)-norketamine (14A) was produced in a similar manner to (S)-(+)-norketamine (2), except using (L)-(S)-(-)-pyro Glutamic acid was used as a chiral resolution reagent instead of (D)-(R)-(+)-pyroglutamic acid. Chiral HPLC: 98% ee. (Chiralpak AD, 60% ethanol in hexane, 1 mL / min, rt: 6.8 min.) [α] D 20 : (-)-75°(c1.0, H 2 O, L-pyroglutamine salt).
Embodiment 3
[0163] Example 3. Synthesis of (S)-tert-butyl (1-(2-chlorophenyl)-2-oxocyclohexyl) carbamate (15)
[0164]
[0165] To a solution of (S)-(+)-norketamine (14) (1.85 g, 8.27 mmol) in toluene (100 mL) was added potassium carbonate (3.43 g, 24.8 mmol) and BOC-anhydride (2.71 g, 12.4 mmol). The reaction was heated to 80 °C and stirred for 16 hours. The reaction was then cooled, extracted with ethyl acetate and washed with water. The organic layer was extracted and the solvent was removed under vacuum to give crude product. Purification by silica gel chromatography (0% to 60% ethyl acetate in hexanes) gave the final product (15) as a white solid.
[0166] 1 H NMR (400MHz, CDCl 3 )δ7.83(d, J=8.0Hz, 1H), 7.42-7.28(m, 2H), 7.28-7.13(m, 1H), 6.59(s, 1H), 3.83(d, J=14.3Hz, 1H ), 2.45-2.36(m, 1H), 2.36-2.25(m, 1H), 2.04(ddq, J=11.5, 5.5, 3.0Hz, 1H), 1.89-1.56(m, 4H), 1.29(s, 9H ).
[0167] 13 C NMR (101MHz, CDCl 3 )δ 209.0, 153.4, 135.1, 133.7, 131.5, 130.9, 129.2, 126.2, 79....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com