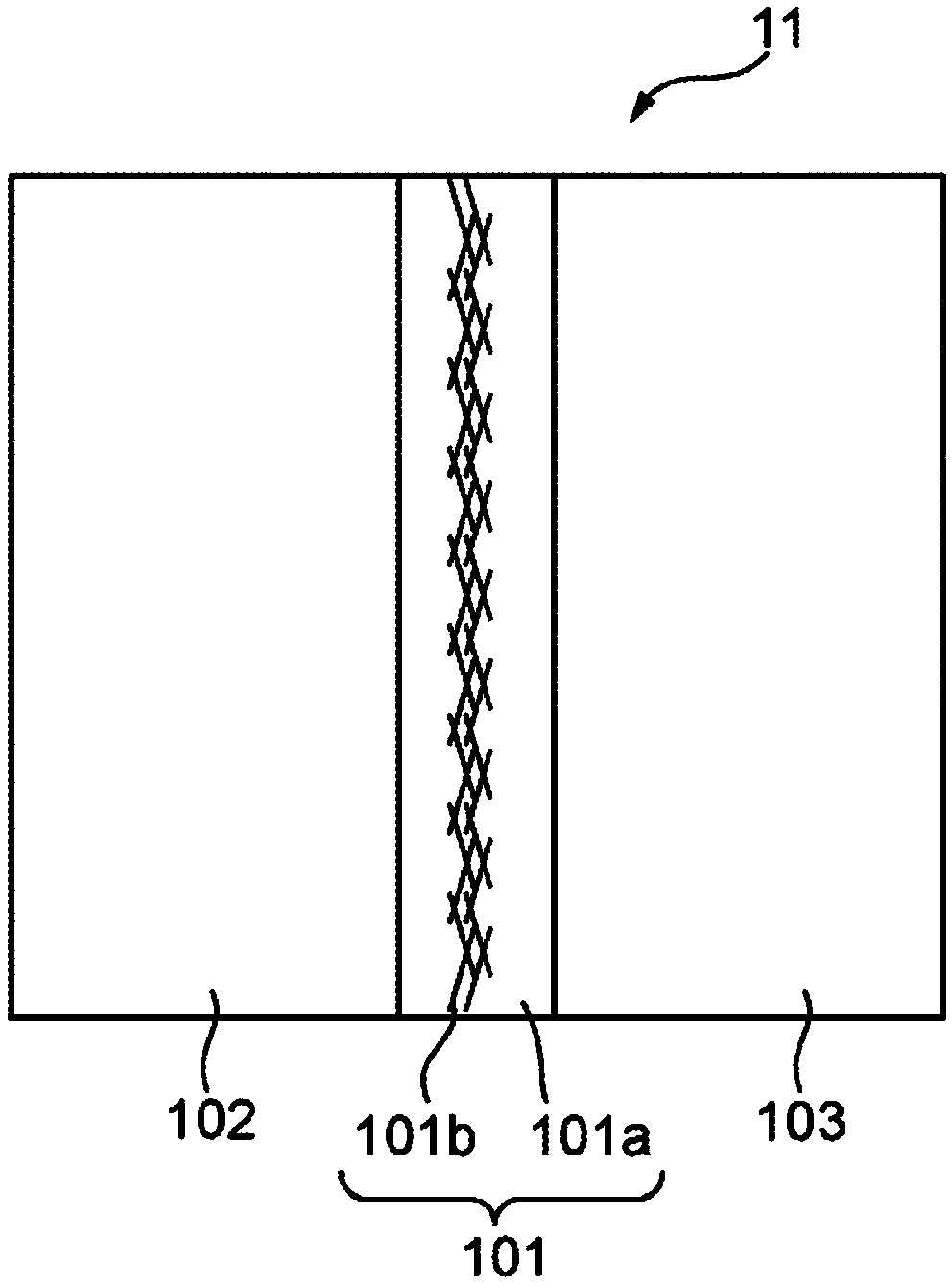
Ion exchange membrane and method of producing same, membrane electrode assembly, and redox flow battery
An ion-exchange membrane and membrane-electrode assembly technology, applied in the field of ion-exchange membranes, can solve problems such as a decrease in current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0079] Hereinafter, exemplary aspects of the present invention will be additionally described using examples, but the present invention is not limited by these examples.
[0080] Ionically Conductive Polymer Dispersions
[0081] The ion-conductive polymer dispersions used are as follows.
[0082] Dispersion 1: Perfluorocarbon sulfonate polymer (sulfonic acid equivalent mass 725, described in U.S. Unexamined Patent Application Publication 2006 / 0014887) in ethanol-water (75 / 25 by mass) mixture A dispersion at a solid concentration of 30% by mass in a dispersion solvent.
[0083] Dispersion 2: Perfluorocarbon sulfonate polymer (sulfonic acid equivalent mass of 825, described in U.S. Unexamined Patent Application Publication 2006 / 0014887) in ethanol-water (mass ratio 75 / 25) mixture A dispersion at a solid concentration of 30% by mass in a dispersion solvent.
[0084] Dispersion 3: Perfluorocarbon sulfonate polymer (sulfonic acid equivalent mass 1000, described in U.S. Unexami...
Embodiment 1 to 10 and comparative example 5 to 9
[0096] For Examples 1 and 8 to 10, Base Film 2 was used, and for Examples 2 to 7, Base Film 1 was used. For Comparative Examples 5 to 6, Base Film 1 was used, and for Comparative Examples 7 to 9, Base Film 2 was used. See Table 1 for the nonwoven fabric used for each sample.
[0097] After electrospinning the specific nonwoven onto the base film (see the corresponding nonwoven and its properties in Table 1 below), the third dispersion was manually coated onto the nonwoven, and the samples were heated at 70°C. Dry for 5 minutes, then dry at 150°C for 10 minutes. Thus, an ion exchange membrane was obtained. The third dispersion used was as follows: Dispersion 5 was used for Examples 1 and 8 to 10, Dispersion 4 was used for Examples 2 to 7, and Dispersion 4 was used for Comparative Examples 5 and 6, and Dispersion 4 was used for Comparative Examples 7 to 6. 9 using dispersion 5.
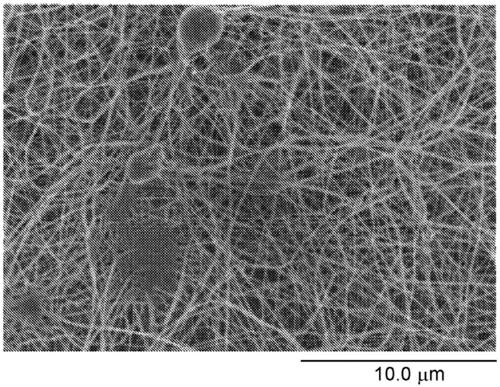
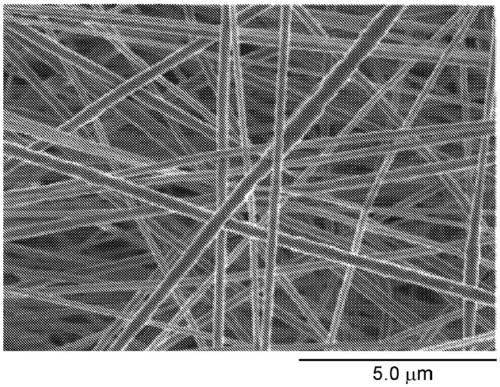
[0098] Figure 2A and Figure 2B Examples of nonwoven fabrics used in Examples and Comparative...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Basis weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com