Novel use of amine hydrochloride compound
A hydrochloride compound and compound technology, applied in the field of compounds, can solve the problems of small number of small molecular compounds and poor specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
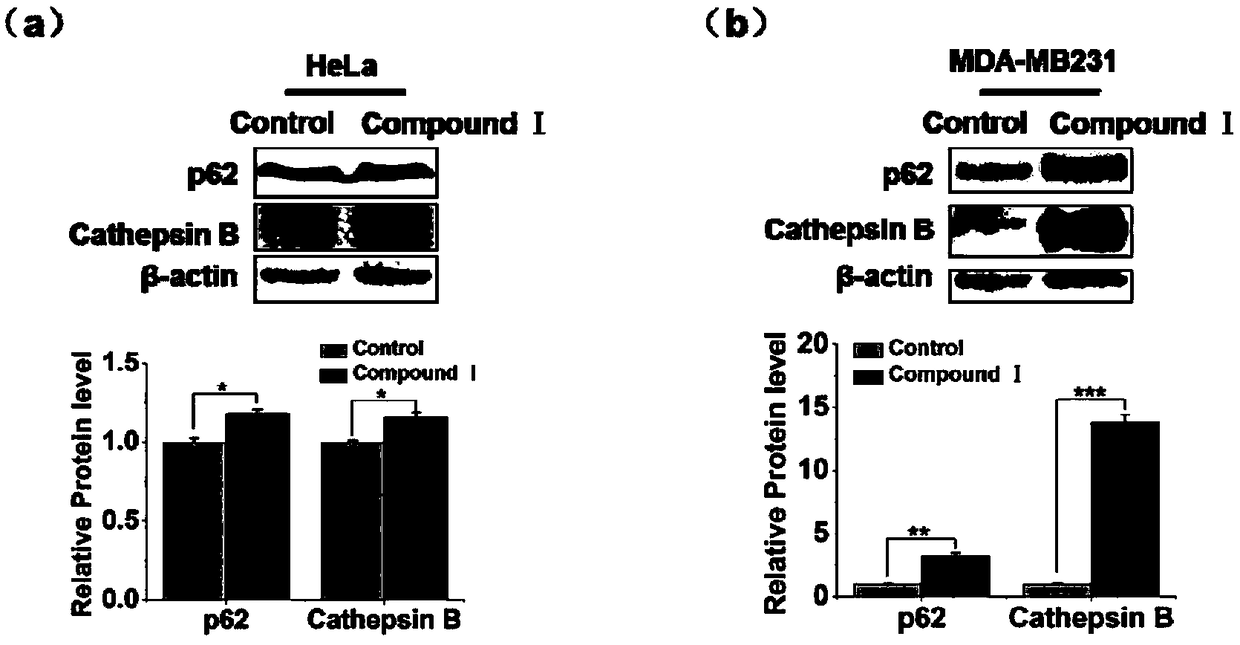
Embodiment 1
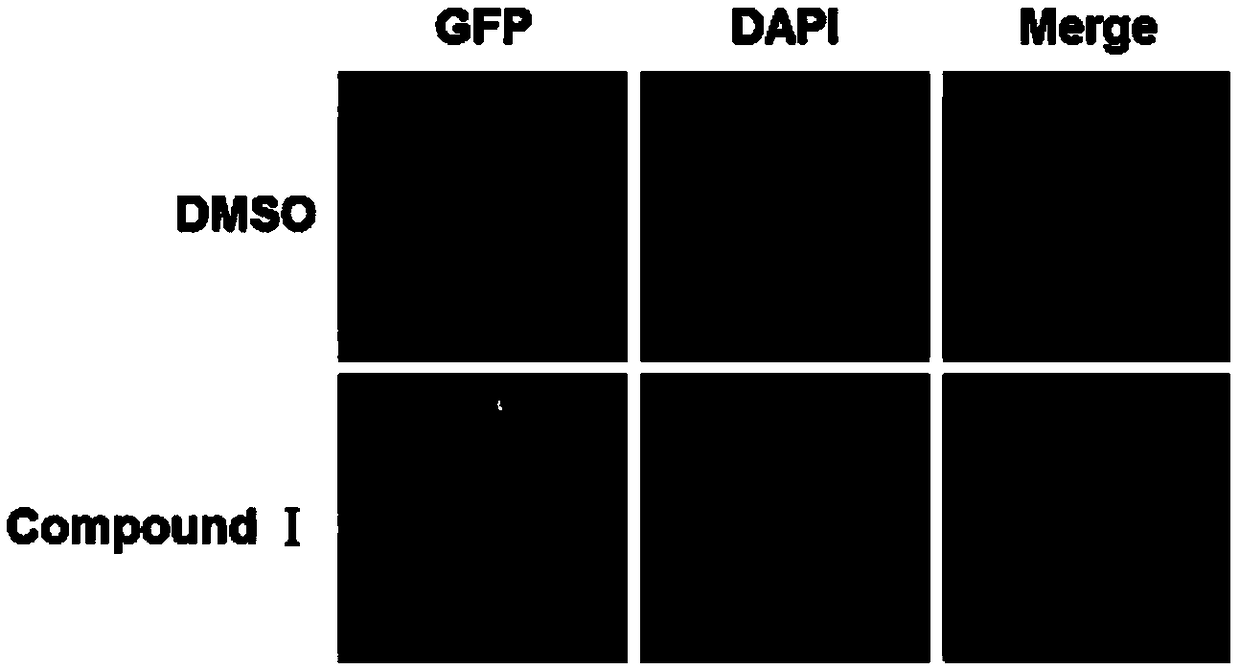
[0032] (1) Screening of drugs that promote TFEB nuclear entry
[0033] ① 96-well plate plating: Collect cells, adjust the concentration of the cell suspension according to the counting results of the hemocytometer, plate to make the density of the cells to be tested 8000 / well, add 200 μL of medium to each well, and fill the edge of the 96-well plate with PBS.
[0034] ②5% CO 2 After incubation at 37° C. for 24 h, drugs (dimethyl sulfoxide (DMSO), compound I) were added at a final concentration of 5 μM.
[0035] ③ After 24 hours of dosing, take pictures with a fluorescence microscope and observe. figure 1 The results showed that compound I could significantly promote the nuclear entry of TFEB-GFP in HeLa cells stably expressing TFEB-GFP.
[0036]
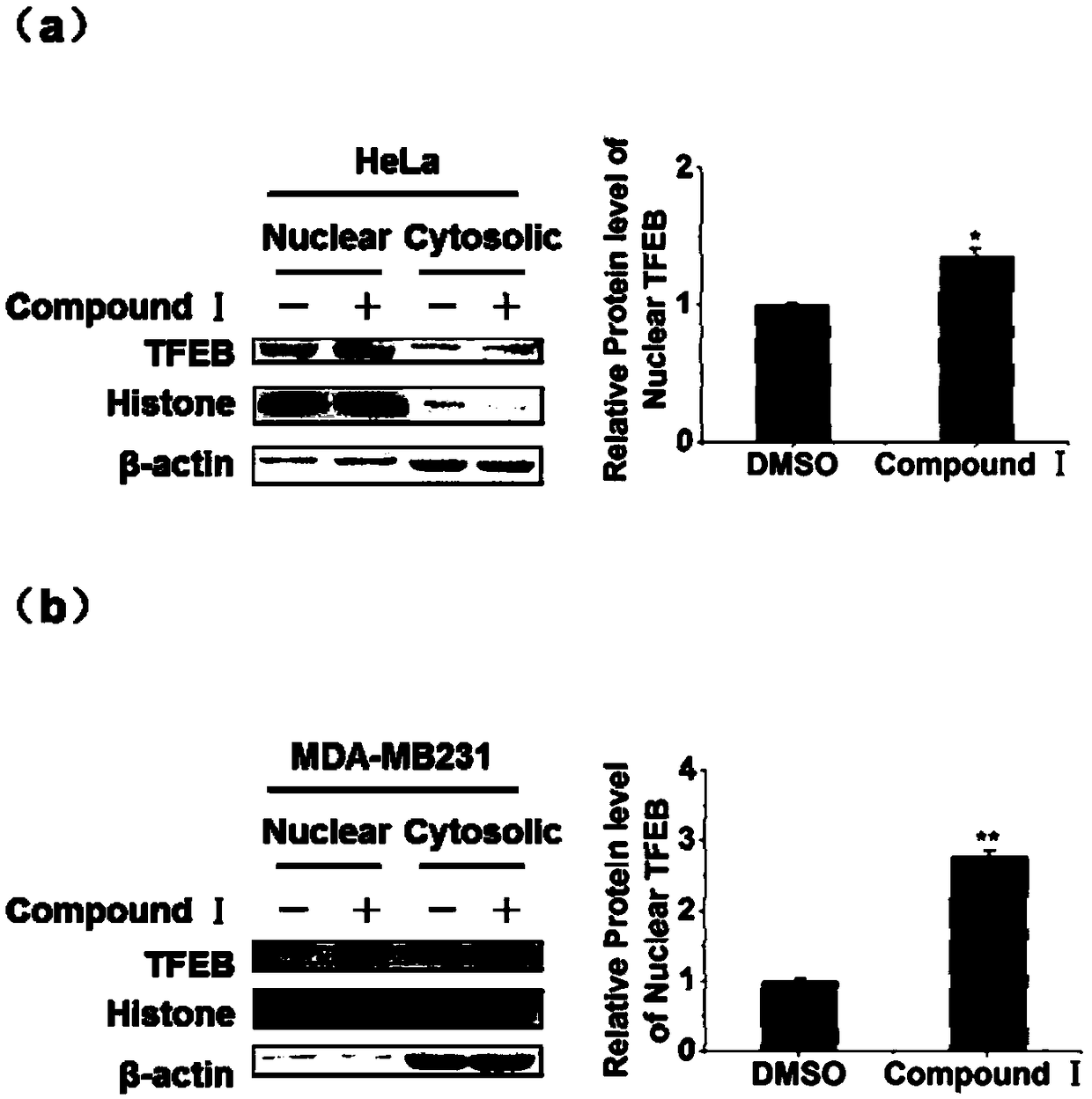
[0037] (2) Nucleoplasmic separation technology
[0038]Using the cytoplasmic protein extraction reagents CERⅠ and CERⅡ, under the condition of low osmotic pressure, the cells are fully expanded, and then the cell membrane is de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com