Application of compound mean in the preparation of anti-hbv medicine
A compound and drug technology, applied in the field of medicine, can solve the problem of unreported antiviral activity, and achieve the effect of strong anti-HBV activity and strong targeting of PTB protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Effects of target compounds on HBV DNA replication
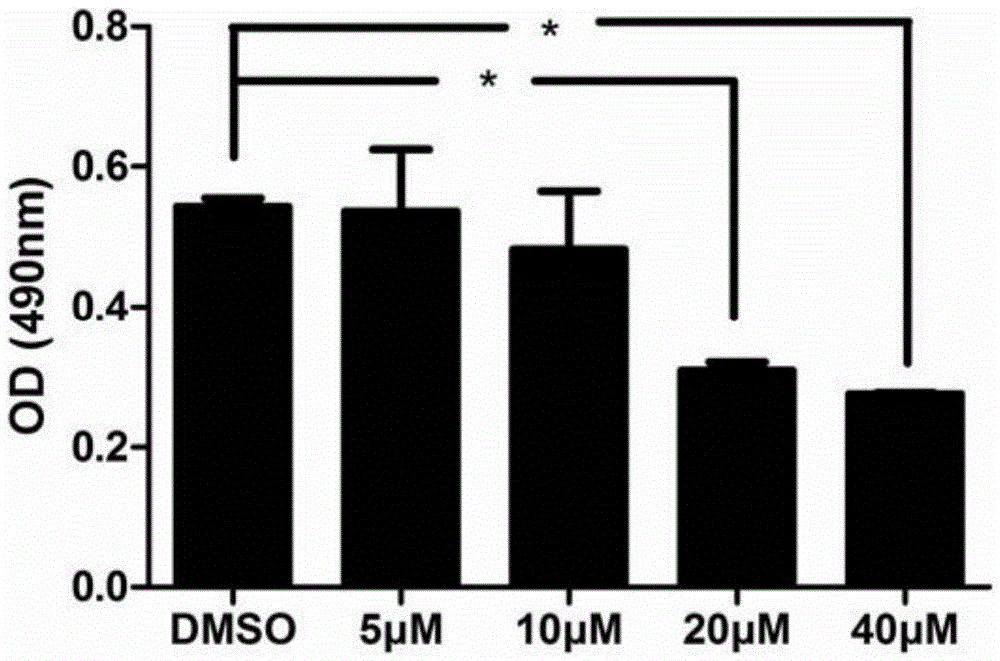
[0034] Such as figure 1 As shown, 40 μM and 20 μM compounds are toxic to HepG2.2.15 cells, 10 μM is not toxic to HepG2.2.15 cells, IC50 is 14.89 μM. Therefore, the concentration of 10 μM or below was used as the intervention concentration to determine the anti-HBV efficacy.
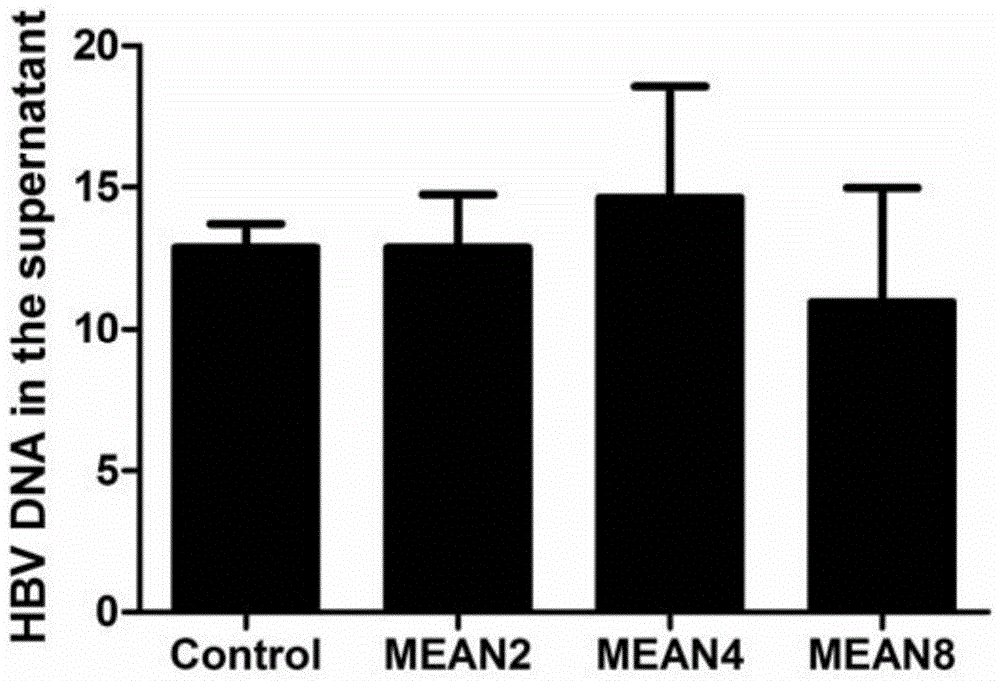
[0035] Compounds were used to intervene HepG2.2.15 cells at concentrations of 8 μM, 4 μM and 2 μM for 2 days, and the level of HBV DNA in the cell supernatant was detected by fluorescent quantitative PCR. The instrument used is ABI7500 real-time fluorescent quantitative PCR system. The specific steps are operated in accordance with the instructions of the Hepatitis B Virus Nucleic Acid Quantitative Detection Kit, and the brief description is as follows: the viral DNA in the cell culture supernatant is extracted with the nucleic acid extraction solution of the kit, and then the viral DNA is used as a template, and the prepared reaction system in...
Embodiment 2
[0038] Effects of target compounds on the secretion and expression of HBeAg
[0039] The compound was intervened in HepG2.2.15 cells at 8 μM, 4 μM and 2 μM concentrations for 2 days, and the HBeAg level in the cell supernatant was detected by electrochemiluminescence technology. The instrument used was Abbott Architect I2000sr (Abbott), and the reagent used was hepatitis B HBeAg matched with the Abbott Architect I2000sr instrument. Quantitative detection manual (Abbott), the specific steps are operated according to the manual.
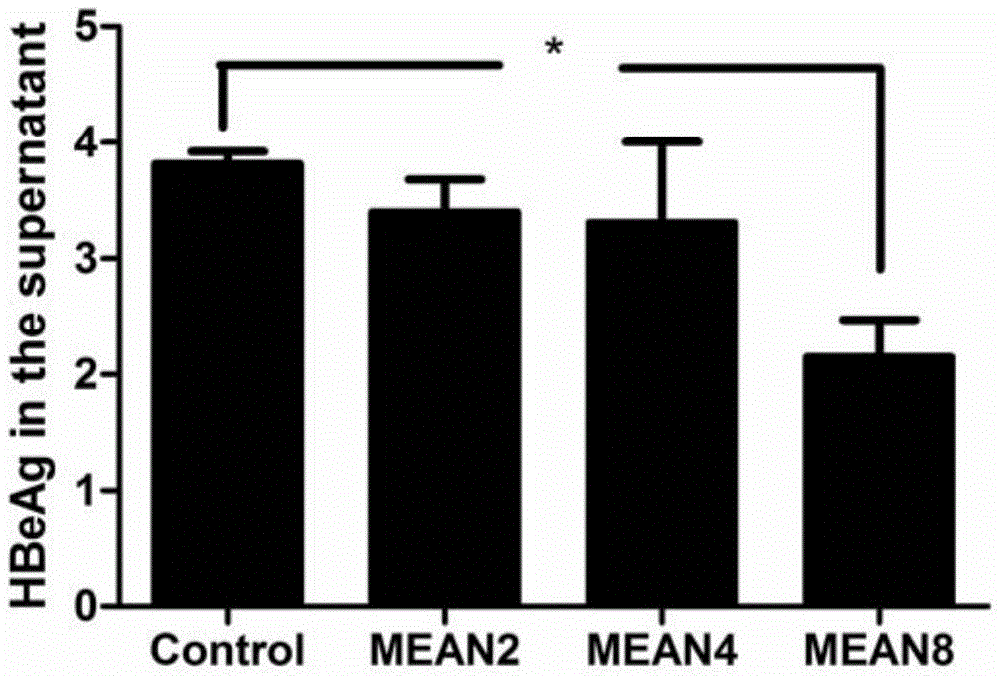
[0040] Such as image 3 As shown, compared with the blank control group, 8 μ M compound MEAN has a significant difference in the inhibitory effect on HBeAg, and other concentrations have no significant difference.
[0041] Effects of target compounds on the secretion and expression of HBsAg
[0042] The compound was intervened in HepG2.2.15 cells at concentrations of 8 μM, 4 μM and 2 μM for 2 days, and the level of HBsAg in the cell supernatant was det...
Embodiment 3
[0045] Effect of target compound on PTB protein expression
[0046] The expression level of PTB protein was detected by Western Blot. Total cell protein was extracted and quantitatively analyzed according to the operating instructions of the BCA protein quantification kit. 40 μg of the above-mentioned total cellular protein was separated by SDS-PAGE and transferred to a nitrocellulose membrane. After blocking with 1% bovine serum albumin, the membrane was incubated with antibodies PTB (self-made) and β-actin (Cellsignalingtechnology) at 4°C overnight, and the secondary antibody was incubated for 1 hour and detected with chemiluminescence reagent ECL (Pierce).
[0047] Such as Figure 5 As shown, compared with the blank control group, MEAN can significantly down-regulate the expression of PTB protein in cells, and there is a significant difference. Different from the literature reports, for RNA viruses, MEAN can inhibit PTB nucleus-cytoplasm translocation in RNA-infected cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com